Article Text
Abstract
Background Daclizumab is an anti-CD25 monoclonal antibody developed for the treatment of relapsing remitting multiple sclerosis, which was withdrawn worldwide in March 2018, due to emerging serious immune-mediated systemic andcentral nervous system adverse events. We report a case of anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis occurring 14 weeks after stopping daclizumab, which responded to the proteasome inhibitor bortezomib.
Methods Following lack of effective clinical response to first line (corticosteroid, plasma exchange, intravenous immunoglobulin) and second line (rituximab) treatments, bortezomib therapy was commenced. The patient received six cycles of bortezomib treatment.
Results Clinical improvement was noted 4 weeks after the first of six cycles of bortezomib and the patient experienced sustained clinical improvement.
Conclusion Our case provides further class IV evidence of the use of bortezomib therapy for treatment refractory anti-NMDAR encephalitis.
- multiple sclerosis
- NMDA
Data availability statement
All data relevant to the study are included in the article.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Daclizumab is a human monoclonal antibody with a number of postulated therapeutic effects in multiple sclerosis (MS). It binds to the interleukin-2 (IL-2) receptor α-chain (CD25) effectively blocking the formation of IL-2 receptor. As IL-2 receptor signalling promotes expansion of activated T cells, by blocking the CD-25 dependent IL-2 pathway, daclizumab selectively inhibits T-cell activation. Additionally daclizumab activates CD56bright natural killer cells which destroy autologous activated T cells and also results in profound inhibition of antigen-specific T cells.1 2
In March 2018, Biogen voluntarily withdrew the marketing authorisation of daclizumab worldwide and the European Medicines Agency announced the suspension and recall of daclizumab in the EU after receiving reports of 12 severe inflammatory CNS disorders in patients on daclizumab.3 Three patients were diagnosed with anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis and at least five of these patient had clinical symptoms consistent with ‘drug rash with eosinophilia and systemic symptoms’ syndrome. We report the occurrence of anti-NMDAR encephalitis, in a patient with relapsing and remitting multiple sclerosis (RRMS), 14 weeks after the discontinuation of daclizumab. Bortezomib therapy (a proteasome inhibitor originally approved for treatment of multiple myeloma) was commenced due to the refractory nature of the illness and promising class IV evidence (with refractory NMDAR encephalitis cases) following which sustained clinical improvement occurred.4
Report of case
A 45-year-old man was diagnosed with RRMS in 2011, with symptom onset 1 year previously. He was enrolled into the DECIDE trial (daclizumab (Zinbryta) vs Interferon beta-1a (Avonex)) from 2012 to 2014, before switching into the open label extension phase with daclizumab, 150 mg once/month s/c (EXTEND trial). Due to the mandated European Medicines Agency (EMA) withdrawal of daclizumab, the patient stopped using this in March 2018 with a plan to switch to Tecfidera (Dimethyl fumarate), after an appropriate washout period.
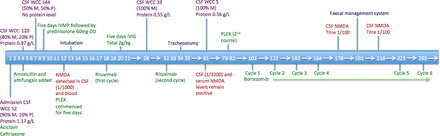
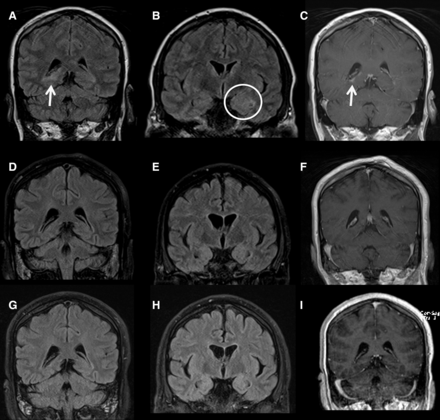
Fourteen weeks after stopping treatment (June 2018), before the planned commencement of Tecfidera, the patient presented to hospital with a 72-hour history of disorientation, fever and two generalised tonic–clonic seizures. He was encephalopathic on admission. An MRI brain scan showed two new demyelinating lesions in the right posterior temporal periventricular white matter and left splenium. There was no leptomeningeal enhancement or changes to suggest immune reconstitution inflammatory syndrome. Cerebrospinal fluid (CSF) analysis revealed 52 leucocytes/µL (90% mononuclear, 10% polymorphs), a protein level of 1.17 g/L and glucose level of 3.0 mmol/L (blood 4.4 mmol/L). Extended viral screen (including JC, West Nile, Toscana) and negative bacterial 16S RNA. Due to worsening encephalopathy over the next 96 hours, two further lumbar punctures were performed (see figure 1). The CSF leucocyte count increased to 144 (50% mononuclear, 50% polymorphs). The patient was commenced on conventional antimicrobial cover for likely CNS infections, with additional listeria and fungal cover. Electroencephalography showed diffuse cortical dysfunction with occasional frontal sharp waves but no delta brush sign. The patient had orofacial movements, episodes of tonic limb posturing, and due to worsening level of consciousness was intubated and ventilated. While awaiting NMDAR antibody results, treatment with one gram pulsed intravenous methylprednisolone was administered for 5 days for presumed autoimmune encephalitis. Following confirmation of positive NMDAR antibodies in CSF and serum, 5 days of plasma exchange were initiated. Due to lack of response and ongoing refractory focal status epilepticus detected on continuous electroencephalogram (EEG) monitoring, rituximab was commenced with the patient receiving two 1 g courses. In addition 2 g/kg intravenous immunoglobulin over 5 days was administered in between rituximab courses. Over the next few weeks continuous EEG monitoring showed sustained refractory seizure activity and the patient required a total of five antiepileptic agents (phenytoin, levetiracetam, diazepam, phenobarbital and perampanel) in addition to propofol and midazolam infusions. CT scan of chest, abdomen and pelvis, whole body fluorodeoxyglucose-positron emission tomography CT and testicular ultrasound did not identify an underlying tumour. Repeat CSF analysis 5 weeks later showed an improvement to 33 leucocytes/µL (100% lymphocytes) and protein level of 0.55 g/L, although there was no improvement in clinical state. Further MRI brain imaging showed bilateral increased mesial temporal lobe signal changes and new right parahippocampal gyrus enhancement on fluid-attenuated inversion recovery sequences (figure 2). A tracheostomy was performed and slow anaesthetic weaning was commenced. There were ongoing orofacial dyskinesias, with intermittent autonomic storming.
Timeline in days illustrating investigation results, treatment and interventions. CSF, cerebrospinal fluid; IVIG, intravenous immunoglobulin; IVMP, intravenous methylprednisolone; M, monocytes; NMDA, N-methyl-D-aspartate; P, polymorphs; PLEX, plasma exchange; WCC, white cell count.
Selected coronal MR images (all 2018) from June (A–C), August (D–F) and November (G–I). FLAIR and postcontrast T1 images in June demonstrate right peritrigonal signal abnormality and enhancement (arrows, A and C). More anterior FLAIR image (B) demonstrates left mesial temporal lobe swelling and signal abnormality (circle) and, to a lesser extent, right temporal and global cerebral swelling. August and November imaging demonstrates a resolution of the peritrigonal abnormality (D and F; G and I) and the temporal lobe and global cerebral swelling, with resulting increased conspicuity of the Sylvian fissures and ventricular system (e and h).
Twelve weeks after onset, and 8 weeks after initiation of rituximab treatment, CSF and serum NMDAR antibody titres had increased despite improvement in basic CSF parameters (5 leucocytes/µL, protein 0.56 g/L) and MRI appearances. Clinical seizures were controlled on multiple antiepileptic agents, but the EEG pattern remained abnormal secondary to ongoing anti-NMDAR encephalitis. The patient remained unresponsive, with intermittent spontaneous eye opening without tracking, ongoing orofacial dyskinesias and daily autonomic storming requiring benzodiazepines. Treatment with bortezomib, a plasma cell depleting agent, was therefore commenced.
One cycle of bortezomib treatment consisted of a 3-week schedule. Subcutaneous injections of 2.5 mg of bortezomib with 20 mg of oral dexamethasone (days 1, 4, 8 and 11), and additional 20 mg of oral dexamethasone (days 2, 5, 9 and 12) were followed by a 10 day rest. He received prophylaxis with daily aciclovir and cotrimoxazole during bortezomib therapy.
Four weeks after the first cycle of bortezomib, clear clinical improvement was noted with cessation of orofacial dyskinesia and autonomic symptoms. The patient started to obey simple commands to movement. Furthermore, although the EEG continued to reflect an encephalopathic state, improvement was noted compared with previous recordings, and no epileptiform activity was identified. CSF cell count normalised to 1 leucocyte/µL although CSF NMDAR levels remained strongly positive. A week following the fourth bortezomib cycle, the patient developed colonic pseudo-obstruction, a described side effect, requiring insertion of a faecal management system.4 5
The fifth cycle of bortezomib was delayed until this improved. Rapid neurological and physical improvement was noted during cycles five and six. The patient demonstrated sustained awareness of his surroundings and was articulating short appropriate sentences via speaking valve.
Shortly following completion of cycle six, tracheostomy and enteral feeding via percutaneous endoscopic gastrostomy were no longer required. At this point, seizure frequency was controlled and allowed for rationalisation of antiepileptic drugs to three agents (levetiracetam, lamotrigine and clobazam).
After a 37-week stay in neurointensive care, the patient was transferred to a ward for neurorehabilitation. As his interactions with staff increased, neuropsychiatric manifestations of anti-NMDAR encephalitis emerged. This comprises Capgras syndrome and unpleasant pseudohallucinations provoked by prolonged eye closure. Initial treatment with risperidone was only partially effective and caused excessive drowsiness, prompting replacement with aripiprazole which he remains on currently. At present, symptoms persist and he is under regular neuropsychiatry review. Cognitively, the patient demonstrates preserved autobiographical memory and problem solving ability, but difficulty with attention and behaviour initiation. Perseveration and delayed recall has also been noted. One year following disease onset, the patient is making a good physical recovery and now mobilises with a frame and supervision of one person. He can perform activities such as dressing and cooking with supervision and verbal prompting. Despite marked clinical improvement, raised CSF NMDAR antibody levels persisted when measured in conjunction with each bortezomib cycle. Serum NMDAR antibody levels have also remained strongly positive.
Discussion
We report a case of anti-NMDAR encephalitis, occurring 14 weeks after the mandated discontinuation of treatment with daclizumab, which had successfully treated RRMS.
Secondary CNS autoimmune complications of daclizumab treatment were first reported to the EMA in February 2018, following which the drug was voluntarily withdrawn in March 2018.3 Although the initial EMA report in March 2018 suggested 12 potential cases of encephalitis or encephalopathy, a revised report in May 2018 identified 9 cases (5 German, 2 American, 1 Swiss and 1 Australian) where the causality of daclizumab could not be ruled out. In one patient, NMDAR antibody was identified. All cases share similar clinical pictures of devastating or fatal outcome (two cases), lack of responses to first line (corticosteroid, plasma exchange, intravenous immunoglobulin) and second line (rituximab) treatments, and unusual findings on brain biopsy.
Our case too shared a similar clinical phenotype to the reported cases and was refractory to standard treatments for antibody mediated immune disorders (including rituximab). Four weeks following the introduction of bortezomib, a proteasome inhibitor particularly effective against immunoglobulin producing plasma cells, clear clinical improvement was observed.
Capgras syndrome has been described in one case of limbic encephalitis, but to our knowledge, not as a feature of NMDAR encephalitis.6 A case of glial fibrillar acidic proteinα immunoglobulin G -associated encephalitis in a patient with MS on daclizumab treatment was reported.1 This patient required treatment with methylprednisolone, plasma exchange and rituximab to achieve clinical stability.
Although anti-NMDAR encephalitis can occur spontaneously, it seems likely that the temporal association with the mandated stopping daclizumab treatment is relevant in our patient. Our patient displayed classical clinical features of anti-NMDAR encephalitis including subacute behavioural changes, orafacial dyskinesia, changes in level of alertness with periods of extreme agitation and generalised seizures. No new anti-NMDAR encephalitis cases off-daclizumab have been reported in the literature or to the EMA (up to 31 December 2020). Additionally, we recommend a low threshold for initiating bortezomib therapy in those refractory to first and second line treatments.
Although development of antibody-mediated immune disorders related to daclizumab is no longer relevant due to the withdrawal of this drug, we feel in this new era of advanced therapies for MS, but also cancer checkpoint-inhibitor therapy and immunotherapies, clinicians need to be aware of a potential increased incidence of autoimmune encephalitis both on and off drug.7 Our case is therefore unique for the delay in onset of anti-NMDAR encephalitis after daclizumab therapy, in combination with the rescue with bortezomib.
Data availability statement
All data relevant to the study are included in the article.
Ethics statements
Acknowledgments
We thank the patient’s next of kin for providing written consent for publication. MSZ has received honoraria from Eisai and UCB Pharma for a lecture each and also supported by the National Brain Appeal but has no financial interests in antibody testing or tests used in this manuscript. JC, MSZ and MPL are supported by Biomedical Research Centre of University College London Hospitals NHS Foundation Trust.
Footnotes
Correction notice This article has been corrected since it was first published. The published version misspelled one of the authors’ names as ‘Bernadette Monoghan’ which has been now amended to ‘Bernadette Monaghan’.
Contributors KK wrote the first draft. TC and TY provided neuroradiology input. DA, BM, MPL, MZ, RSH, DMK, JS, MW and JC edited the draft. JC is senior author and did final manuscript edit.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.