Abstract
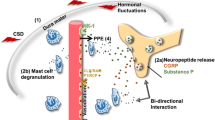
Migraine is considered one of the most prevalent neurological disorders but its underlying pathophysiology is poorly understood. Over the past two decades, it became widely accepted that activation of primary afferent nociceptive neurons that innervate the intracranial meninges serves as a key process that mediates the throbbing head pain of migraine. Knowledge about the endogenous factors that play a role in promoting this neural process during a migraine attack slowly begins to increase, and a better understanding remains one of the holy grails in migraine research. One endogenous process, which has been invoked as a major player in the genesis of migraine pain, is cortical spreading depression (CSD). Until recently, however, this notion was only supported by indirect evidence. Recently, electrophysiological data provided the first direct evidence that CSD is indeed a powerful endogenous process that can lead to persistent activation of meningeal nociceptors and the migraine pain pathway. CSD has been suggested to promote persistent sensitization and ensuing activation of meningeal nociceptors through a mechanism involving local neurogenic inflammation including the activation of mast cells and macrophages and subsequent release of inflammatory mediators. Local action of such nociceptive mediators can increase the responsiveness of meningeal nociceptors. Recent studies provided key experimental data implicating complex meningeal immuno-vascular interactions, in particular, the interplay between proinflammatory cytokines, the meningeal vasculature and immune cells, in enhancing the responses of meningeal nociceptors.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193–210.
Moskowitz MA. Defining a pathway to discovery from bench to bedside: the trigeminovascular system and sensitization. Headache. 2008;48(5):688–90.
Olesen J, Burstein R, Ashina M, Tfelt-Hansen P. Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol. 2009;8(7):679–90.
Levy D, Strassman AM, Burstein R. A critical view on the role of migraine triggers in the genesis of migraine pain. Headache. 2009;49(6):953–7.
Messlinger K. Migraine: where and how does the pain originate? Experimental brain research Experimentelle Hirnforschung. 2009;196(1):179–93.
Strassman AM, Levy D. Response properties of dural nociceptors in relation to headache. J Neurophysiol. 2006;95(3):1298–306.
Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130(1–2):166–76.
Levy D, Kainz V, Burstein R, Strassman AM. Mast cell degranulation distinctly activates trigemino-cervical and lumbosacral pain pathways and elicits widespread tactile pain hypersensitivity. Brain Behav Immun. 2011 Oct 12.
Zhang XC, Levy D. Modulation of meningeal nociceptors mechanosensitivity by peripheral proteinase-activated receptor-2: the role of mast cells. Cephalalgia. 2008;28(3):276–84.
Zhang XC, Strassman AM, Burstein R, Levy D. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322(2):806–12.
Dalkara T, Zervas NT, Moskowitz MA. From spreading depression to the trigeminovascular system. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. [Review]. 2006 May;27 Suppl 2:S86-90.
Ebersberger A, Schaible HG, Averbeck B, Richter F. Is there a correlation between spreading depression, neurogenic inflammation, and nociception that might cause migraine headache? Ann Neurol. 2001;49(1):7–13.
Goadsby PJ. Migraine, aura, and cortical spreading depression: why are we still talking about it? Annals of neurology [Comment Editorial]. 2001;49(1):4–6.
Levy D. Migraine pain, meningeal inflammation, and mast cells. Curr Pain Headache Rep. 2009;13(3):237–40.
Perini F, D’Andrea G, Galloni E, Pignatelli F, Billo G, Alba S, et al. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45(7):926–31.
Sarchielli P, Alberti A, Baldi A, Coppola F, Rossi C, Pierguidi L, et al. Proinflammatory cytokines, adhesion molecules, and lymphocyte integrin expression in the internal jugular blood of migraine patients without aura assessed ictally. Headache. 2006;46(2):200–7.
Rozen T, Swidan SZ. Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache. 2007;47(7):1050–5.
Junger H, Sorkin LS. Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain. 2000;85(1-2):145–51.
Constantin CE, Mair N, Sailer CA, Andratsch M, Xu ZZ, Blumer MJ, et al. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28(19):5072–81.
Leem JG, Bove GM. Mid-axonal tumor necrosis factor-alpha induces ectopic activity in a subset of slowly conducting cutaneous and deep afferent neurons. J Pain. 2002;3(1):45–9.
Hakim AW, Dong XD, Svensson P, Kumar U, Cairns BE. TNFalpha mechanically sensitizes masseter muscle afferent fibers of male rats. J Neurophysiol. 2009;102(3):1551–9.
•• Zhang XC, Kainz V, Burstein R, Levy D. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain. 2010 Oct 29. This is an important paper describing the complex meningeal immuno-vascular interaction that mediates the nociceptive action of TNF-α in the meninges.
Levy D. Migraine pain and nociceptor activation–where do we stand? Headache. 2010;50(5):909–16.
Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361(1–3):184–7.
Li Y, Ji A, Weihe E, Schafer MK. Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor alpha (TNFalpha) and TNF receptors in rat dorsal root ganglion. J Neurosci. 2004;24(43):9623–31.
Cunha TM, Verri Jr WA, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci USA. 2005;102(5):1755–60.
Schafers M, Sommer C, Geis C, Hagenacker T, Vandenabeele P, Sorkin LS. Selective stimulation of either tumor necrosis factor receptor differentially induces pain behavior in vivo and ectopic activity in sensory neurons in vitro. Neuroscience. 2008;157(2):414–23.
Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121(3):417–24.
Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107(3):660–4.
Poole S, Cunha FQ, Selkirk S, Lorenzetti BB, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-10. Br J Pharmacol. 1995;115(4):684–8.
Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17(9):1847–52.
Schafers M, Marziniak M, Sorkin LS, Yaksh TL, Sommer C. Cyclooxygenase inhibition in nerve-injury- and TNF-induced hyperalgesia in the rat. Exp Neurol. 2004;185(1):160–8.
Bowen EJ, Schmidt TW, Firm CS, Russo AF, Durham PL. Tumor necrosis factor-alpha stimulation of calcitonin gene-related peptide expression and secretion from rat trigeminal ganglion neurons. J Neurochem. 2006;96(1):65–77.
Mackay F, Loetscher H, Stueber D, Gehr G, Lesslauer W. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J Exp Med. 1993;177(5):1277–86.
Zhang XC, Kainz V, Jakubowski M, Burstein R, Strassman A, Levy D. Localization of COX-1 and COX-2 in the intracranial dura mater of the rat. Neurosci Lett. 2009;452(1):33–6.
Elmquist JK, Breder CD, Sherin JE, Scammell TE, Hickey WF, Dewitt D, et al. Intravenous lipopolysaccharide induces cyclooxygenase 2-like immunoreactivity in rat brain perivascular microglia and meningeal macrophages. J Comp Neurol. 1997;381(2):119–29.
Levy D, Zhang XC, Jakubowski M, Burstein R. Sensitization of meningeal nociceptors: inhibition by naproxen. Eur J Neurosci. 2008;27(4):917–22.
Modur V, Zimmerman GA, Prescott SM, McIntyre TM. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J Biol Chem. 1996;271(22):13094–102.
Ferrero E, Zocchi MR, Magni E, Panzeri MC, Curnis F, Rugarli C, et al. Roles of tumor necrosis factor p55 and p75 receptors in TNF-alpha-induced vascular permeability. Am J Physiol Cell Physiol. 2001;281(4):C1173–9.
Zauli G, Pandolfi A, Gonelli A, Di Pietro R, Guarnieri S, Ciabattoni G, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) sequentially upregulates nitric oxide and prostanoid production in primary human endothelial cells. Circ Res. 2003;92(7):732–40.
Leao AA. Spreading depression of activity in cerebral cortex. J Neurophysiol. 1944;7:359–90.
Grafstein B. Locus of propagation of spreading cortical depression. J Neurophysiol. 1956;19(4):308–16.
• Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ. Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab. 2011 Jan;31(1):17-35. This is an excellent review on the relevance of cortical spreading depression to migraine and other neurological disorders.
Fabricius M, Fuhr S, Bhatia R, Boutelle M, Hashemi P, Strong AJ, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129(Pt 3):778–90.
Mayevsky A, Doron A, Manor T, Meilin S, Zarchin N, Ouaknine GE. Cortical spreading depression recorded from the human brain using a multiparametric monitoring system. Brain research [Research Support, Non-US Gov’t]. 1996;740(1-2):268–74.
Strong AJ, Fabricius M, Boutelle MG, Hibbins SJ, Hopwood SE, Jones R, et al. Spreading and synchronous depressions of cortical activity in acutely injured human brain. Stroke. 2002;33(12):2738–43.
Milner PM. Note on a possible correspondence between the scotomas of migraine and spreading depression of Leão. Electroencephalogr Clin Neurophysiol. 1958;10(4):705.
Olesen J, Larsen B, Lauritzen M. Focal hyperemia followed by spreading oligemia and impaired activation of rCBF in classic migraine. Ann Neurol. 1981;9(4):344–52.
Lauritzen M, Olesen J. Regional cerebral blood flow during migraine attacks by Xenon-133 inhalation and emission tomography. Brain. 1984;107(Pt 2):447–61.
Hadjikhani N, Sanchez Del Rio M, Wu O, Schwartz D, Bakker D, Fischl B, et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex. Proc Natl Acad Sci USA. 2001;98(8):4687–92.
Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984;16(2):157–68.
Lauritzen M. Pathophysiology of the migraine aura. The spreading depression theory. Brain: a journal of neurology [Research Support, Non-US Gov’t Review]. 1994;117(1):199–210.
Moskowitz MA, Nozaki K, Kraig RP. Neocortical spreading depression provokes the expression of c-fos protein-like immunoreactivity within trigeminal nucleus caudalis via trigeminovascular mechanisms. J Neurosci. 1993;13(3):1167–77.
Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8(2):136–42.
•• Zhang X, Levy D, Noseda R, Kainz V, Jakubowski M, Burstein R. Activation of meningeal nociceptors by cortical spreading depression: implications for migraine with aura. J Neurosci. 2010 Jun 30;30(26):8807-14. This paper provides the first documented direct evidence linking CSD to persistent activation of meningeal nociceptors.
Zhang X, Levy D, Kainz V, Noseda R, Jakubowski M, Burstein R. Activation of central trigeminovascular neurons by cortical spreading depression. Ann Neurol. 2011;69(5):855–65.
Nozaki K, Boccalini P, Moskowitz MA. Expression of c-fos-like immunoreactivity in brainstem after meningeal irritation by blood in the subarachnoid space. Neuroscience. 1992;49(3):669–80.
Strassman AM, Mineta Y, Vos BP. Distribution of fos-like immunoreactivity in the medullary and upper cervical dorsal horn produced by stimulation of dural blood vessels in the rat. J Neurosci. 1994;14(6):3725–35.
Busija DW, Bari F, Domoki F, Horiguchi T, Shimizu K. Mechanisms involved in the cerebrovascular dilator effects of cortical spreading depression. Prog Neurobiol [Research Support, NIH, Extramural Research Support, Non-US Gov’t Review]. 2008;86(4):379–95.
Scheckenbach KE, Dreier JP, Dirnagl U, Lindauer U. Impaired cerebrovascular reactivity after cortical spreading depression in rats: Restoration by nitric oxide or cGMP. Exp Neurol. 2006;202(2):449–55.
Chang JC, Shook LL, Biag J, Nguyen EN, Toga AW, Charles AC, et al. Biphasic direct current shift, haemoglobin desaturation and neurovascular uncoupling in cortical spreading depression. Brain. 2010;133(Pt 4):996–1012.
Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, et al. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10(6):754–62.
Piilgaard H, Lauritzen M. Persistent increase in oxygen consumption and impaired neurovascular coupling after spreading depression in rat neocortex. J Cereb Blood Flow Metab. 2009;29(9):1517–27.
Ingvardsen BK, Laursen H, Olsen UB, Hansen AJ. Possible mechanism of c-fos expression in trigeminal nucleus caudalis following cortical spreading depression [see comments]. Pain. 1997;72(3):407–15.
Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol. 1993;33(1):48–56.
Moskowitz MA. Pathophysiology of headache–past and present. Headache. 2007;47 Suppl 1:S58–63.
Moskowitz MA. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology. 1993;43(6 Suppl 3):S16–20.
Alvaro G, Di Fabio R. Neurokinin 1 receptor antagonists–current prospects. Curr Opin Drug Discov Devel. 2007;10(5):613–21.
Levy D, Burstein R, Strassman AM. Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: Implications for the pathophysiology of migraine. Ann Neurol. 2005;58(5):698–705.
Wahl M, Schilling L, Parsons AA, Kaumann A. Involvement of calcitonin gene-related peptide (CGRP) and nitric oxide (NO) in the pial artery dilatation elicited by cortical spreading depression. Brain Res. 1994;637(1–2):204–10.
Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. Nature. 1996;384(6609):560–4.
Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci USA. 2004;101(12):4274–9.
Gursoy-Ozdemir Y, Qiu J, Matsuoka N, Bolay H, Bermpohl D, Jin H, et al. Cortical spreading depression activates and upregulates MMP-9. The Journal of clinical investigation [Research Support, Non-US Gov’t Research Support, US Gov’t, PHS]. 2004;113(10):1447–55.
Olesen J, Lauritzen M, Tfelt-Hansen P, Henriksen L, Larsen B. Spreading cerebral oligemia in classical- and normal cerebral blood flow in common migraine. Headache. 1982;22(6):242–8.
Burstein R, Jakubowski M, Levy D. Anti-migraine action of triptans is preceded by transient aggravation of headache caused by activation of meningeal nociceptors. Pain. 2005;115(1–2):21–8.
Brennan K, Charles A. An update on the blood vessel in migraine. Current opinion in neurology. 2010 Mar 4.
Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431(7005):195–9.
Scotland RS, Chauhan S, Davis C, De Felipe C, Hunt S, Kabir J, et al. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: a novel mechanism involved in myogenic constriction. Circulation research [Research Support, Non-US Gov’t]. 2004;95(10):1027–34.
Shinohara M, Dollinger B, Brown G, Rapoport S, Sokoloff L. Cerebral glucose utilization: local changes during and after recovery from spreading cortical depression. Science. 1979;203(4376):188–90.
Amery WK. Migraine and cerebral hypoxia: a hypothesis with pharmacotherapeutic implications. Cephalalgia. 1985;5 Suppl 2:131–3.
Schoonman GG, Sandor PS, Agosti RM, Siccoli M, Bartsch P, Ferrari MD, et al. Normobaric hypoxia and nitroglycerin as trigger factors for migraine. Cephalalgia. 2006;26(7):816–9.
Appenzeller O. High-altitude headache. Cephalalgia. 1994;14(5):317–8.
Bartsch P, Maggi S, Kleger GR, Ballmer PE, Baumgartner RW. Sumatriptan for high-altitude headache. Lancet. 1994;344(8934):1445.
Myers DE, Myers RA. A preliminary report on hyperbaric oxygen in the relief of migraine headache. Headache. 1995;35(4):197–9.
Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz MA. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59(4):652–61.
Ayata C. Spreading depression: from serendipity to targeted therapy in migraine prophylaxis. Cephalalgia. 2009;29(10):1095–114.
Silberstein SD. Preventive treatment of migraine. Trends Pharmacol Sci. 2006;27(8):410–5.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Levy, D. Endogenous Mechanisms Underlying the Activation and Sensitization of Meningeal Nociceptors: The Role of Immuno-Vascular Interactions and Cortical Spreading Depression. Curr Pain Headache Rep 16, 270–277 (2012). https://doi.org/10.1007/s11916-012-0255-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11916-012-0255-1