Abstract
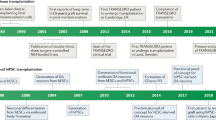
Clinical studies of Parkinson’s disease (PD) using a dopamine cell replacment strategy have been tried for more than 30 years. The outcomes following transplantation of human fetal ventral mesencephalic tissue (hfVM) have been variable, with some patients coming off their anti-PD treatment for many years and others not responding and/or developing significant side effects, including graft-induced dyskinesia. This led to a re-appraisal of the best way to do such trials, which resulted in a new European-Union-funded allograft trial with fetal dopamine cells across several centers in Europe. This new trial, TRANSEURO (NCT01898390), is an open-label study in which some individuals in a large observational cohort of patients with mild PD who were undergoing identical assessments were randomly selected to receive transplants of hfVM. The TRANSEURO trial is currently ongoing as researchers have completed both recruitment into a large multicenter observational study of younger onset early-stage PD and transplantation of hfVM in 11 patients. While completion of TRANSEURO is not expected until 2021, we feel that sharing the rationale for the design of TRANSEURO, along with the lessons we have learned along the way, can help inform researchers and facilitate planning of transplants of dopamine-producing cells derived from human pluripotent stem cells for future clinical trials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Goedert, M., Spillantini, M. G., Del Tredici, K. & Braak, H. 100 years of Lewy pathology. Nat. Rev. Neurol. 9, 13–24 (2013).
Schapira, A. H. V., Chaudhuri, K. R. & Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 509 (2017).
Barker, R. A., Drouin-Ouellet, J. & Parmar, M. Cell-based therapies for Parkinson disease — past insights and future potential. Nat. Rev. Neurol. 11, 492–503 (2015).
Palfi, S. et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson’s disease: a dose escalation, open-label, phase 1/2 trial. Lancet 383, 1138–1146 (2014).
Freed, C. R. et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 344, 710–719 (2001).
Olanow, C. W. et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 54, 403–414 (2003).
Barker, R. A., Barrett, J., Mason, S. L. & Bjorklund, A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson’s disease. Lancet Neurol. 12, 84–91 (2013).
Kirkeby, A., Parmar, M. & Barker, R. A. Strategies for bringing stem cell-derived dopamine neurons to the clinic: A European approach (STEM-PD). Prog. Brain. Res. 230, 165–190 (2017).
Liu, G. et al. Prediction of cognition in Parkinson’s disease with a clinical-genetic score: a longitudinal analysis of nine cohorts. Lancet Neurol. 16, 620–629 (2017).
Politis, M. et al. Serotonergic neurons mediate dyskinesia side effects in Parkinson’s patients with neural transplants. Sci. Transl. Med. 2, 38ra46 (2010).
Piccini, P. et al. Factors affecting the clinical outcome after neural transplantation in Parkinson’s disease. Brain 128, 2977–2986 (2005).
Piccini, P. et al. Delayed recovery of movement-related cortical function in Parkinson’s disease after striatal dopaminergic grafts. Ann. Neurol. 48, 689–695 (2000).
Piroth, T. et al. Transplantation of human fetal tissue for neurodegenerative diseases: validation of a new protocol for microbiological analysis and bacterial decontamination. Cell Transplant. 23, 995–1007 (2014).
Rath, A. et al. Survival and functional restoration of human fetal ventral mesencephalon following transplantation in a rat model of Parkinson’s disease. Cell Transplant. 22, 1281–1293 (2013).
Kelly, C. M. et al. Medical terminations of pregnancy: a viable source of tissue for cell replacement therapy for neurodegenerative disorders. Cell Transplant. 20, 503–513 (2011).
Kordower, J. H. et al. Functional fetal nigral grafts in a patient with Parkinson’s disease: chemoanatomic, ultrastructural, and metabolic studies. J. Comp. Neurol. 370, 203–230 (1996).
Li, W. et al. Extensive graft-derived dopaminergic innervation is maintained 24 years after transplantation in the degenerating parkinsonian brain. Proc. Natl Acad. Sci. USA 113, 6544–6549 (2016).
Brundin, P. et al. Bilateral caudate and putamen grafts of embryonic mesencephalic tissue treated with lazaroids in Parkinson’s disease. Brain 123, 1380–1390 (2000).
Kefalopoulou, Z. et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 71, 83–87 (2014).
Fahn, S. et al. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 351, 2498–2508 (2004).
Carpenter, B. et al.Stan: a probabilistic programming language. J. Stat. Softw. 76, https://doi.org/10.18637/jss.v076.i01 (2017).
Bürkner, P. brms: An R Package for Bayesian Multilevel Models Using Stan. J. Stat. Softw. 80, https://doi.org/10.18637/jss.v080.i01 (2017).
Li, W. et al. (11) C-PE2I and (18) F-Dopa PET for assessing progression rate in Parkinson’s: A longitudinal study. Mov. Disord. 33, 117–127 (2018).
Lindvall, O. et al. Transplantation of fetal dopamine neurons in Parkinson’s disease: one-year clinical and neurophysiological observations in two patients with putaminal implants. Ann. Neurol. 31, 155–165 (1992).
Lindvall, O. et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. A detailed account of methodology and a 6-month follow-up. Arch. Neurol. 46, 615–631 (1989).
Laguna Goya, R., Busch, R., Mathur, R., Coles, A. J. & Barker, R. A. Human fetal neural precursor cells can up-regulate MHC class I and class II expression and elicit CD4 and CD8 T cell proliferation. Neurobiol. Dis. 41, 407–414 (2011).
Liu, X., Li, W., Fu, X. & Xu, Y. The immunogenicity and immune tolerance of pluripotent stem cell derivatives. Front. Immunol. 8, 645 (2017).
Scheiner, Z. S., Talib, S. & Feigal, E. G. The potential for immunogenicity of autologous induced pluripotent stem cell-derived therapies. J. Biol. Chem. 289, 4571–4577 (2014).
Galpern, W. R. et al. Sham neurosurgical procedures in clinical trials for neurodegenerative diseases: scientific and ethical considerations. Lancet Neurol. 11, 643–650 (2012).
Acknowledgements
The authors acknowledge D. Marsden, N. Cawthorne and T. Stone from the Cambridge University Hospital Clinical Engineering Innovation (CEI) who helped in the development of the in house delivery device in Cambridge, and K. Richardson, K. Jestice, and M. Scott from the Cambridge Cellular Therapy Laboratory, Cambridge University Hospitals NHS Foundation Trust who helped in developing the SOPs for the hfVM tissue preparations in Cambridge.
This study was supported by an EU FP7 grant (242003) as well as funding from the Cure Parkinson’s Trust (RG81537), John Black Charitable Trust and Multipark. The work was also supported by NIHR funding of Biomedical Research Centres at UCL and Cambridge (146281). R.A.B. is an NIHR Senior Investigator (NF-SI-0616-10011) and a PI in the MRC/WT Stem Cell Institute (203151/Z/16/Z).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
R.A.B. advises Living Cell Technologies, FujiFilm Cellular Dynamics Inc; BlueRock Therapeutics; Novo Nordisk; Sana Therapeutics and Cellino Biotech on their cell-based therapies for PD as well as UCB, Roche and Lundbeck on other aspects of neurodegenerative disorders of the brain. T.P. has received honorary fees from Boston Scientific for peer-to-peer workshops (related to DBS) in 2018 and 2019. T.F. advises Living Cell Technologies on their cell-based therapy, as well as Bial, Profile Pharma, Peptron and Boston Scientific on other aspects of neurodegenerative disorders of the brain. A.B. is a consultant for Novo Nordisk. M.P. is the owner of Parmar Cells AB and co-inventor on US patent applications 15/093,927 owned by Biolamina AB and EP17181588 owned by Miltenyi Biotec. Patent WO 2015/114059 A1 patents the use of BCL2 in reprogramming. S.P. advises Oxford Biomedica and Axovant
Additional information
Peer review information: Joao Monteiro was the primary editor on this perspective and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barker, R.A., TRANSEURO consortium. Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat Med 25, 1045–1053 (2019). https://doi.org/10.1038/s41591-019-0507-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-019-0507-2