Article Text
Abstract
Objective Continuous electroencephalography (cEEG) is increasingly used to detect non-convulsive seizures in critically ill patients but is not widely practised in Australasia. Use of cEEG is also influencing the management of status epilepticus (SE), which is rapidly evolving. We aimed to survey Australian and New Zealand cEEG use and current treatment of SE
Methods A web-based survey was distributed to Epilepsy Society of Australia (ESA) members, between October and November 2019. Adult and paediatric neurologists/epileptologists with ESA membership involved in clinical epilepsy care and cEEG interpretation were invited to participate.
Results Thirty-five paediatric/adult epileptologists completed the survey, 51% with over 10 years of consultant experience. cEEG was always available for only 31% of respondents, with the majority having no or only ad hoc access to cEEG. Lack of funding (74%) and personnel (71%) were the most common barriers to performing cEEG. Although experience with SE was common, responses varied regarding treatment approaches for both convulsive and non-convulsive SE. Escalation to anaesthetic treatment of convulsive SE tended to occur later than international guideline recommendations. There was general agreement that formal training in cEEG and national guidelines for SE/cEEG were needed.
Conclusions cEEG availability remains limited in Australia, with lack of funding and resourcing being key commonly identified barriers. Current opinions on the use of cEEG and treatment of SE vary reflecting the complexity of management and a rapidly evolving field. An Australian-based guideline for the management of SE, including the role of cEEG is recommended.
- electroencephalography
- epilepsy
- anticonvulsants
- intensive care
- neurophysiol
- clinical
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Assessment and management of status epilepticus (SE) and urgent electroencephalography (EEG) review is an essential component of emergency neurology and epileptology. Use of continuous EEG (cEEG) to guide diagnosis and management of SE and non-convulsive seizures is increasing worldwide.1 The identification of seizures, which are predominantly non-convulsive (or subclinical), in a substantial proportion of critically ill patients, and the recognition of the contribution of these seizures to morbidity and mortality has attracted significant attention over the last decade.2 cEEG is defined as a prolonged recording and allows non-invasive real-time measurement of brain electrophysiological activity in critically ill patients in whom clinical assessment is unreliable, typically in the intensive care unit (ICU). However, cEEG is highly labour and resource intense, and requires specialised expertise and training of medical, EEG neuroscientist and nursing staff. Two pivotal large database studies, 1 of 41 000 unselected ventilated adult patients in the ICU and the other involving over 7 million ICU patients of whom 22 700 had cEEG, showed that the use of cEEG was associated with a significantly improved mortality compared with just using routine EEG in age-matched and illness-matched patients.1 3 However, a prospective European study of 364 patients with altered consciousness randomised to cEEG or repeated routine EEG, showed no between-group differences in mortality at 6 months; notably, this study excluded patients who experienced a seizure within 36 hours of the EEG, and included a high proportion of patients with hypoxic ischaemic encephalopathy, a group that rarely requires cEEG.4
The mortality risk of SE is substantial, and becomes even higher once medically refractory, beyond the effect of the underlying aetiology. A recent review showed poor long-term outcomes after SE, with mortality rates reaching 20% for children and 55% in adults.5 The most recent definition of SE from the International League Against Epilepsy provides a time ‘t1’ for when seizures are prolonged where normal mechanisms that serve to terminate seizures fail, and a time ‘t2’ in which long-term consequences are likely to occur.6 Akin to hyperacute stroke treatment, the concept of ‘time is brain’ is increasingly used for SE to stress the importance of urgent cessation of seizures and the avoidance of subsequent morbidity and mortality associated with ongoing seizures. The use of cEEG use for the assessment and management of convulsive SE (CSE) and non-convulsive SE (NCSE), excluding seizure mimics where unnecessary and dangerous escalation of care including intubation may occur, has also been recommended in specific settings7 8 and should be included in guidelines for SE The overall cost–benefit of cEEG guided management of SE, taking into account improvements in morbidity and mortality, is unknown.9
Various international guidelines exist for the assessment and management of SE, but are lacking in the Australian context.10 A recent systematic review from Australian authors concluded that there are often deviations from published within hospital guidelines of SE, and that non-adherence to these guidelines is associated with worse outcomes.11 Significantly improved outcomes in seizure cessation, necessity for ICU admission and length of stay, have been demonstrated with adherence to treatment protocols.12 While neurologists and epileptologists may not always be involved in the initial management of CSE, their input is invariably needed in NCSE; thus, their involvement in the development of treatment guidelines and use of cEEG is required.
The current Australian landscape of cEEG and treatment of SE is unknown, on both an individual health service and state levels. This study sought to capture current clinical practices in Australia and to assess opinions of practising clinicians in the Australian epilepsy community.
Methods
Design
A web-based survey of cEEG use and management of SE was distributed via electronic mailing lists.
Population
Clinicians who are members of the Epilepsy Society of Australia (ESA), which includes some practising in New Zealand, were invited to complete the survey between October and November 2019. Participation was strictly voluntary, and all responses were anonymous. A decision to commence the survey indicated consent to use any data provided for the purposes described.
Questions
Survey questions were designed by the investigators, aiming to capture information on respondent demographics and level of experience, fellowship training in epilepsy, college affiliation, participants’ availability and use of urgent and cEEG, and experience in managing CSE and NCSE (see online supplemental material).
Supplemental material
Data analysis
Descriptive analysis was undertaken and compared with available guidelines.
Results
A total of 43 responses were obtained between October and November 2019. Of these, 7 incomplete responses, defined as less than half the questions completed, and one respondent who was exclusively ICU trained were excluded. Therefore, 35 responses were included in the analysis. One respondent did not complete the cEEG questions and another did not complete SE management questions.
Demographics and expertise of respondents
Characteristics of respondents are shown in table 1. Most were males. The sample comprised adult (66%) and paediatric (31%) neurologists/epileptologists from all Australian states (n=33) and New Zealand (n=2). Expertise displayed a bimodal distribution of both early career (n=9, 30%) but predominantly experienced physicians (n=18, 60%), of whom eighteen self-identified as epileptologists. Membership across the Royal Australasian College of Physicians, Australian and New Zealand Association of Neurologists (ANZAN) and ESA was almost universal. Level 3 ANZAN EEG board certification was common (54%), with most having done at least 1 year of epilepsy fellowship. Ninety-four per cent were involved in reporting cEEG.
Characteristics of respondents
Self-reported confidence was high in the diagnosis of NCSE, as well as the management of NCSE and CSE. The majority of respondents felt that there should be formal teaching in cEEG (n=33, 94%), and most agreed that Australian cEEG guidelines should be developed (n=26, 74%). Individual comments regarding cEEG training centred on importance of recognition of clinically significant EEG patterns, cEEG training being taught alongside traditional EEG training, and provision for critical care EEG fellowships.
Facilities, equipment and personnel
All respondents had access to an ICU, but less than half to a neurohigh-dependency unit (n=15, 43%; table 2). cEEG was only available on an ad hoc basis for just over half (n=19, 54%), always available to some (n=11, 31%), with routine EEG only available in a smaller group (n=5, 14%). There were similarly split proportions of neuroscientist coverage to set up the cEEG with 24 hours on-call availability for 9 (26%), 7 days in-hours only for 11 (31%) and 5 days a week for 15 (43%). Rostered on-call 24 hours epileptologist coverage was available for 26%; 20% reported unrostered and unpaid 24 hours cover and the remainder provided epileptology or neurology cover for EEG within standard hours or less. Twenty-eight respondents had remote access to EEG (n=28, 80%), with 57% having access to live real-time recordings. Review of EEG was largely performed on an ad hoc basis (n=21, 60%), with only few utilising quantitative EEG (QEEG) (n=4, 11%). Lack of funding (n=26, 74%) and personnel (n=25, 71%) were the most consistently reported barriers to the practice of cEEG, with lack of physical resources (n=12, 34%) also identified as an important barrier. Five respondents felt there was a lack of evidence for cEEG (n=5, 14%).
Facilities, equipment and personnel
Initial management of SE
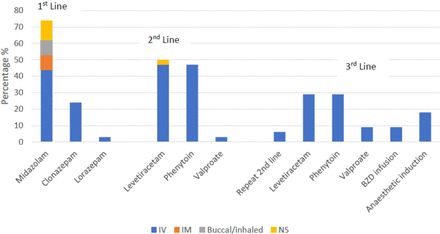
The most common initial treatment of SE was intravenous midazolam (n=25, 73%), see figure 1. Clonazepam was the second choice for first-line therapy. Second-line therapy was split evenly between levetiracetam (n=17, 50%) and phenytoin (n=16, 47%), with one response for valproate. Interestingly, for third line therapy levetiracetam and phenytoin were the most common (29% each) reflecting movement from each of the groups for second line to the alternate medication. Only 18% of respondents indicated that they would use anaesthetic induction if there were ongoing seizures after administration of a benzodiazepine and the second line agent. Although specific dosing information was asked for each of the lines of therapy, responses were highly variable and therefore not reported here.
Initial management of SE. BZD, benzodiazepine; NS, not specified.
Subsequent management of SE
Sixty-five per cent of respondents indicated they would advise administration of an anaesthetic following failure of the second antiepileptic drug (AED), with 65% applying this approach for CSE and 50% for NCSE (see online supplemental material). Only 26% of respondents would advise treatment with an anaesthetic and intubation following first AED and second line failure for CSE. Nine per cent would never advise the use of an anaesthetic for NCSE. There were varied comments regarding anaesthetic induction for NCSE, with most suggesting that repeated trials of other AEDs may be required and that the approach should be tailored to the clinical situation. Propofol (n=24, 73%) followed closely by midazolam (n=21, 64%) were the most commonly reported anaesthetics recommended. Barbiturates were less commonly preferred. ICU management including intubation was accepted as maximal therapy in nearly half of respondents for NCSE (n=16, 48%). In the setting of a poor recovery following offset of an overt seizure and when suspecting NCSE, most would recommend an EEG within an hour (n=30, 88%) and some after 10 min (n=5, 15%). There was a wide range of responses regarding the required EEG duration when assessing for non-convulsive seizures, with thirty-eight percent suggesting a 24-hour recording and 24% a 1-hour recording only. Treatment targets for refractory SE also varied, with some suggesting a target of electrographic seizure cessation (n=10, 30%) and others would aim for a 24-hour or greater period of burst suppression (n=9, 27%). Seizure cessation defined via cEEG was preferred for NCSE by 48% of respondents. Seventy-eight per cent of respondents were in favour of national guidelines for SE
Discussion
Use of cEEG
This study surveyed practices of experienced paediatric and adult epileptologists from Australia and New Zealand, who are at the forefront of decision making in the management of SE and use of cEEG. The findings are comparable to other similarly themed international surveys.13 Substantial barriers to cEEG were identified, with un-rostered, unfunded and resource-limited work leading to a restricted ability to offer cEEG services to a wider patient group. Epilepsy services and cEEG are also typically limited to major metropolitan hospitals. Lack of physical resourcing of EEG machines and remote access to live recordings, limited personnel availability including scientist coverage, and physician time to report the studies are also significant issues.
The identification of non-convulsive seizures requires the use of cEEG. The accurate diagnosis of non-convulsive seizures and NCSE is challenging, requiring experienced cEEG trained personnel, and is context specific with some clinical situations having a high pretest probability of identifying non-convulsive seizures. Therefore, specificity may be preferable to sensitivity when determining whom to test, especially when establishing a cEEG service. Although published recommendations may include a range of potential indications for cEEG,7 14 this must be balanced on the available resources and equity for patients. Careful attention to patient selection and screening EEG duration are important practical considerations, that may improve pretest probability. Epileptiform abnormalities seen in the first 30 min of a recording may help predict sensitivity of detecting seizures,15 16 representing a practical step in algorithm flow of EEG assessment. A recent study developed a scoring system, the 2HELPS2B score, to predict seizures in this patient group to help triage cEEG services.17
Real-time cEEG ‘monitoring’, rather than retrospective cEEG ‘review’, requires a dynamic setup including remote and live review capacity and at least twice daily review and reporting.7 A minimum set of technical requirements are needed to adequately and safety perform cEEG.8 In centres without a dedicated neurology specific ICU, the provision of cEEG is the responsibility of the neurology department. Close communication with ICU staff regarding the EEG results and suggested management changes is imperative which should be supervised by cEEG trained epileptologists. QEEG is an emerging EEG trend analysis software with the ability for rapid EEG review and reasonably accurate automated seizure detection.18 QEEG is an underused tool that may significantly reduce the workload associated with 24-hour raw EEG review using a compressed timescale, and trend guided analysis alongside referencing with the raw EEG. Other avenues of allowing a more rapid and user-friendly EEG assessment are adaptation of standard EEG montages with a reduction in electrodes. The balance of lower resolution and maintenance of seizure detection accuracy is essential, and there are some devices already in development.
Management of SE
The management of SE is a time critical emergency with outcomes improved by early cessation of seizures, necessitating a clear understanding of principles to guide evidence-based rapid algorithmic care. There are no unified Australian guidelines or consensus within the national context, although some specific hospital guidelines exist. International guidelines from Europe,19 UK20 and USA21 are variable and may not be appropriate for use in Australia given the different availability of drugs in our country. There is no consensus in the literature regarding choice of the first-line benzodiazepine with diazepam, lorazepam and clonazepam appearing in the different guidelines; despite its widespread use, intravenous midazolam has never been adequately tested but may be safely given in the prehospital setting via intramuscular, intrabuccal or intranasal routes. Clonazepam is often used in epilepsy monitoring units to terminate prolonged seizures and has been used in one prehospital trial of early SE,22 but is not commonly used in Australian emergency departments. Inadequate dosing and overuse of benzodiazepines leading to respiratory failure and intubation, and a delay to second-line therapy have previously been highlighted.11
Second-line treatment of established SE was essentially evenly split evenly between phenytoin and levetiracetam in this survey, which reflects the current literature, with the trend towards the newer and easier to us levetiracetam, which has been recently shown to be non-inferior to phenytoin in both children and adults.23–25 Levetiracetam treatment failure in the past may have been attributable to significant underdosing. Valproate remains an equally efficacious alternative,23 but was not a preferred choice in our survey.
Provided that adequate dosing of an AED as second-line therapy is achieved, adding a further alternative AED may delay anaesthetic induction and intubation as recommended for refractory convulsive SE in international guidelines.21 Anaesthetic preference varied in this survey, although propofol and midazolam remain most popular, reflecting the literature and the widespread experience with these drugs. Treatment of NCSE diverges at this point from recent recommendations suggesting that aggressive management via ICU treatment may lead to poorer outcomes. The use of cEEG is pivotal here, first in establishing the diagnosis of SE, but also in evaluating treatment response. Urgent EEG assessment of an unresponsive patient with suspected non-convulsive seizures or SE is recommended. Treatment aggressiveness of non-convulsive seizures and NCSE is often the topic of debate at international conferences,26 although recent evidence from dynamic neurophysiological changes and outcome studies are supporting the role of suppression of seizures for a neuroprotective benefit.9 Treatment targets vary from seizure suppression to burst suppression, although in some cases in order to achieve the first aim burst suppression may be required. These complex decisions often arise on a case-by-case basis, considering concurrent active medical issues and the state of the patient. Various treatments exist in super-refractory SE with limited evidence due to the lack of controlled studies or even large-scale cohort studies. Recognition of the association of autoimmune encephalitis underlying a large proportion of new-onset refractory SE is essential, as immunotherapy may be indicated and can significantly improve outcomes.27
Limitations
Participant numbers, or responder rate, in this survey was relatively low which is comparable to other physician web-based surveys. The number of subspecialist epileptologists in Australia is not known. At the time of the survey, there were 188 ESA members whom were specified as clinicians in neurology. Therefore, a 19% (35/188) responder rate overall, including a 28% (30/108) responder rate specifically for consultant neurologists/epileptologists was deemed reasonable. Considering the spread of respondents from different states, this is also reflective of the few hospitals with specialist epilepsy services within Australia that have the capacity for cEEG and lead the management of SE.
Conclusion
This study shows characterisation of adoption of cEEG in Australia, and the current practices in managing SE, identifying key barriers to further implementation of cEEG and a variable approach to SE Given the experienced subspecialist epileptologists surveyed in this study, there is likely further substantial variation in SE management in non-speciality centres. Ongoing engagement with the Australian epilepsy and critical care communities will aid in understanding gaps in the use of cEEG and in the treatment of SE, ultimately leading to the development of appropriate Australian guidelines.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors JL designed the study with assistance from NL, PP, PK and TJO. JL conducted the recruitment, data collection and analysis. All authors critically revised the manuscript and contributed to the final submission and review process.
Funding JL is funded by an NHMRC PhD Scholarship.
Disclaimer The funding body has no influence over the preparation or content of this paper.
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This study was approved by the Low Risk Subcommittee of the Human Research and Ethics Committee of Monash University (project ID 21673).
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement All data relevant to the study are included in the article or uploaded as online supplemental information.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.