Article Text
Abstract
Background Since 2014, the USA has documented three outbreaks of acute flaccid myelitis (AFM). Unique features and treatment responses of this myelitis variant have not been prospectively studied. This study prospectively measured outcomes in paediatric myelitis patients relative to treatments.
Methods This was a prospective, multicentre, non-randomised, observational cohort study. The study duration was 5 years and the length of follow-up was 1 year. This study collected data from children and families in North America. Patients were enrolled at academic centres with expertise in myelitis or online via a web portal. Paediatric patients diagnosed with myelitis were eligible for enrolment in the study within 6 months of onset of symptoms. Patients were characterised as transverse myelitis (TM) or the AFM variant based on clinical and radiographic findings.
Results The cohort of 90 patients included patients with AFM and TM. Of the 51 patients with AFM there was evidence of two clinically relevant patterns. This included a grey matter restricted form of AFM and a cohort with concomitant white matter that could explain lower extremity motor deficits in patients with lesions restricted to the cervical spine. The improvement in deficits with the use of corticosteroids was similar to what was observed in the TM cohort (p=0.97).
Conclusions Clinicians should consider on a case by case basis the approach to therapy for AFM patients. Prospective controlled studies of long-term outcomes would be useful in this growing patient population.
- myelopathy
- paediatric neurology
Data availability statement
Data are available on reasonable request. Data can be provided to researchers with IRB approved/exempt protocols on request and review.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Introduction
Transverse myelitis (TM) has been classically described as an immune mediated condition causing demyelination within the spinal cord.1 This term has caused confusion for clinicians, patients and researchers. Pathologically, any inflammation of the spinal cord (regardless of cause) can be called myelitis. When patients present with flaccid weakness in at least one limb and MRI changes predominate in the grey matter, patients are diagnosed with Acute Flaccid Myelitis (AFM). This subtype of myelitis has been increasingly recognised within the USA since 2014.2 Significant data suggest an association between this condition and enteroviral infections, with over 500 confirmed cases among children since 2014.3 The clinical and radiographic findings are quite similar to poliomyelitis caused by the poliovirus, with some distinct differences. Early in the course of these outbreaks the Center for Disease Control published treatment guidelines counselling clinicians to avoid corticosteroid and plasma exchange (PLEX) therapy due to a concern about worsening viral infections within the spinal cord (which have since been updated).2 During this time, the Patient-Centered Outcomes Research Institute funded CAPTURE (Collaboration About Paediatric Transverse myelitis: Understand, Reveal, Educate) Study was prospectively enrolling paediatric myelitis patients into a study of treatment outcomes. While the CAPTURE study collected outcomes from traditional TM and AFM patients, this paper outlines novel findings relative to the AFM cohort. The CAPTURE database was analysed to determine if there was any positive or negative impact to the use of corticosteroids or PLEX in this unique patient population. The foundation of TM treatment has included corticosteroids and PLEX so the data from this cohort was used to place outcome data from the AFM patients into context.
Methods
Seven enrolling centres were designated in the United States (Children’s Health in Dallas, Texas; University of Colorado in Denver, Colorado; Toronto Sick Kids in Toronto, Canada; Children’s Hospital of Philadelphia in Philadelphia, Pennsylvania, Johns Hopkins Hospital in Baltimore, Maryland, Kennedy Krieger Institute in Baltimore, Maryland; Cincinnati Children’s in Cincinnati, Ohio). Potential research participants were recruited by contacting the TM association, a patient advocacy organisation or by contacting an enrolling centre. Informed consent was obtained from parents of study participants and assent was obtained from participants 10 and older at an enrolling centre or by the central recruiting programme in Dallas, Texas, prior to the initiation of any study procedures.
Participants who were able to travel to an enrolling centre were entered into the ‘in-person’ cohort where they completed visits to obtain clinician-derived data. They also submitted patient-reported and parent-reported outcomes via electronic surveys at defined time points after symptom onset. Participants who were unable to travel were entered into the ‘virtual’ cohort in which they only submitted the electronic surveys. Subjects enrolled in the virtual cohort had imaging and medical records reviewed at the University of Texas Southwestern for case determination and classification. Study data were collected and managed using the REDCap (Research Electronic Data Capture) tools hosted at The University of Texas(UT) Southwestern.4
Study cohort
Participants were eligible for enrollment if they were diagnosed with myelitis, were between the ages of 0 and 17 at diagnosis, within 6 months of symptom onset, had access to the internet and had parents/guardians that were able to provide informed consent. Patients with diagnoses of systemic autoimmune conditions were excluded. If a patient had no flaccid weakness and predominantly white matter involvement, then they were classified as TM. Patients were classified as AFM if they had one or more flaccid limbs and predominantly grey matter changes on MRI. AFM patients were categorised as grey matter restricted if the T2 hyperintense signal change on MRI was only identified in anterior horns vs mixed grey and white matter if the T2 hyperintense signal change involved both grey and white matter.
Study period
The study initially launched in 2014 and continued through 2018. Patients were followed through the 1-year postonset time point.
Outcomes
For all patients enrolled, medical records and MRIs were examined to record date of onset; symptoms at onset; location of symptoms (right/left and upper/lower extremity); the presence of bowel and bladder symptoms; treatment(s) at onset; degree-of-improvement after each treatment; laboratory results; past illnesses, immunisations, family history and radiologic findings. Radiographic assessments and classifications were conducted centrally at UT Southwestern in a blinded fashion to the clinical data.
Longitudinal outcomes included patient and parent-reported outcomes and clinician-derived outcomes obtained within 30 days of symptom onset, 3 months postonset, 6 months postonset and 1-year postonset. Due to the inclusion criteria of the study allowing for patients who were at most 6 months postonset, many enrollees were missing data from the time of symptom onset or 3 months postonset.
The longitudinal patient-reported outcomes were collected via REDCap surveys distributed to patients and included the anger, anxiety, depression, fatigue, mobility, pain interference, peer relations, upper extremity function and paediatric quality of life PROMIS (Patient-Reported Outcomes Measurement Information System) forms.5 6 Additionally, the PROMIS Parent Proxy forms were collected. The clinician derived data included 25-foot walk time, 6 min walk, Hauser Ambulation Index, FIM/WeeFIM (V.4.0). The FIM/WeeFIM (range 18–126) was the originally defined primary outcome while PROMIS scores were a secondary outcome. The PROMIS Paediatric and Parent Proxy short forms consist of questions resulting in a set of ordinal responses with lower scores equating to more severe symptoms. The PROMIS Paediatric and Parent Proxy forms included the following short forms: (1) emotional distress—anxiety (eight ordinal responses); (2) emotional distress—depressive symptoms (six ordinal responses); (3) fatigue (10 ordinal responses); (4) pain—interference (eight ordinal responses); (5) peer relationships (seven ordinal responses); (6) physical function—mobility (eight ordinal responses) and (7) physical function—upper extremity (eight ordinal responses). The PROMIS paediatric forms are only valid for patients 8 years of age and older and PROMIS Parent Proxy forms are only valid for patients between the ages of 5 and 17 years old.
The longitudinal clinician-derived data were collected for those subjects enrolled in the ‘in-person’ cohort and included 25-foot walk time, 6 min walk, Hauser Ambulation Index, WeeFIM (V.4.0).
If corticosteroids led to a worsening of the viral infection within the spinal cord, it would be expected that AFM patients who received corticosteroids would have a worse prognosis relative to AFM patients who did not receive corticosteroids and relative to TM patients who did receive corticosteroids.
Enterovirus testing
PCR testing was performed on cerebrospinal fluid (CSF), nasopharyngeal swab or stool specimens.
Missing data
Data were missing due to four causes: (1) participants enrolled 30 days after symptom onset or 30 days after 3 months postonset; (2) patients’ failure to complete REDCap surveys within ±30 days of symptom onset, 3 months postonset, 6 months postonset and/or 1-year postonset; (3) patients’ failure to adhere to clinic visit schedule and (4) failure of clinicians to capture all relevant data during visits.
Statistical analysis
Demographic and clinical characteristics were compared between the ‘in-person’ and ‘virtual’ cohorts to determine if it was reasonable to pool the data from these two cohort. Categorical data were compared using two-tailed Fisher’s exact tests and ordinal and continuous variables were compared using the Mann-Whitney U test.
After determining there was no evidence of differences between the ‘in-person’ and ‘virtual’ cohorts, the data were pooled for comparison of TM and AFM, as well as the subtypes of AFM. Two-tailed Fisher’s exact tests were used to compare ORs between AFM and TM patient cohorts (or between grey matter isolated AFM and mixed-matter AFM). Ordinal logistics regression was used to compare the degree-of-improvement after initial treatment between AFM patients who received corticosteroids as their initial treatment versus those who received intravenous immunoglobulin as their initial treatment.
Significance was defined as 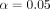 . Multiple testing adjustments were not used due to the exploratory nature of this analysis. However, it must be noted that because multiple testing adjustments were not performed, the reader must interpret all significant results with caution due to the inflation of a type I error and future efforts designed to answer defined hypotheses must be pursued to provide more definitive inferences. It should be noted that P25 denotes the 25th percentile and P75 denotes the 75th percentile. All data analyses were performed in R (V.3.6.1).
. Multiple testing adjustments were not used due to the exploratory nature of this analysis. However, it must be noted that because multiple testing adjustments were not performed, the reader must interpret all significant results with caution due to the inflation of a type I error and future efforts designed to answer defined hypotheses must be pursued to provide more definitive inferences. It should be noted that P25 denotes the 25th percentile and P75 denotes the 75th percentile. All data analyses were performed in R (V.3.6.1).
Results
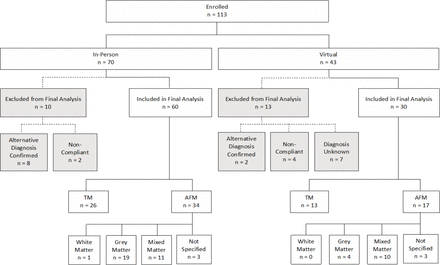
A total of 113 patients were initially enrolled in the CAPTURE study. Figure 1 provides a depiction of those patients excluded and included in the final analysis. There were 90 enrollees with analysable data (figure 1). Patients were excluded because of missing data relative to diagnosis (n=7), lack of follow-up (n=6) or alternate diagnoses (n=10). Fifty-one was classified as AFM and 39 as TM based on clinical and radiographic findings. The data stratified by cohort membership, diagnosis and AFM subtype are provided in online supplemental eTables 1 and 2.
Supplemental material
Enrolment diagram for the capture study. AFM, acute flaccid myelitis; TM, transverse myelitis.
Demographic and clinical data
Demographic and clinical data for the TM and AFM cohorts, as well as AFM stratified by MRI pattern are summarised in table 1. Based on the results in table 1, of the 36 TM patients and 49 AFM patients with data related to the presence (or absence) of a preceding illness, 63.9% of TM patients reported a preceding illness, while 81.6% of the AFM patients had an illness in the 90 days prior to symptom onset (OR 0.40, 95% CI 0.13 to 1.20, p=0.08), with 30 (60%) of them reporting a respiratory illness and 9 (18%) reporting a gastrointestinal/diarrhoeal illness. Forty-five (88.2%) of the AFM patients had no documented vaccinations within 90 days of onset.
Demographic, symptoms at onset and laboratory results; cells without sample sizes denote results obtained from all patients in the given cohort
AFM clinical data
At onset, more than half of AFM patients had weakness of the upper extremities, which was greater than the third of TM (38.5%) patients reporting upper extremity weakness at onset (OR=0.39, 95% CI 0.15 to 0.98, p=0.03). Of the AFM patients, 19 (38%) had upper extremity symptoms only, 12 (24%) had both upper and lower extremity symptoms and 17 (34%) had lower extremity symptoms only. Fifteen (30%) AFM patients had sensory symptoms and 19 (38%) had bowel or bladder symptoms at onset (table 1).
Within the AFM patient population, CSF studies revealed a mean protein of 69.6 dg/mL (SD 96.5) and a mean white blood cell count of 37.4 white blood cells/mm3 (SD 53.0). Oligoclonal bands were absent in all cases for which data were available (table 1).
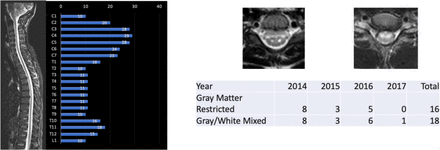
MRI findings revealed 68% of AFM cases had a lesion that involved C4 compared with 43.2% of TM cases (OR 0.36, 95% CI 0.14 to 0.94), p=0.03). Review of axial imaging was possible in 45 AFM cases (six cases were not available for central review, but were classified as AFM based on their radiology report) and revealed two predominant patterns of cord involvement: lesions restricted to grey matter 23 (51.1%) and lesions that involved both the grey and white matter 21 (46.7%).(figure 2) (table 2) Of the 45 patients with axial imaging available for review, 40 (88.9%) had contiguous lesions and five had two separate lesions. Six of the 23 AFM (26.1%) patients with lesions restricted to grey matter had abnormalities restricted to the thoracic cord or lower, while this number was 6 of 21 (28.6%) among the patients with mixed involvement of both grey and white matter (table 1). For those patients with lesions restricted to the C3–C5 spinal segments, 93.5% had upper extremity weakness relative to the 31.6% of patients with lesions outside of the C3–C5 segments (OR 28.35, 95% CI 4.7 to 321.0), p<0.001).
CAPTURE study AFM cohort MRI characteristics: two types identified. AFM, acute flaccid myelitis; CAPTURE, Collaboration About Paediatric Transverse myelitis: Understand, Reveal, Educate.
MRI results by diagnosis; cells without sample sizes denote results obtained from all patients in the given cohort
Viral data
Fifty of the 51 AFM patients had data available relative to verifying the performance or lack of performance of enterovirus testing (one patient had missing data). PCR testing was performed on CSF, nasopharyngeal swab or stool specimens. Eleven of 50 AFM patients with recorded data indicated that no testing occurred. Of the 39 AFM patients who had a verifiable specimen tested for enteroviruses, 18 tested positive (46.2%). Seventeen of these positives were from nasopharyngeal swabs. Restricting to patients who had respiratory samples tested for enteroviruses, the percent positive among AFM patients was 51.5%. No patients had a positive CSF PCR for Enteroviruses (table 1). Of note, three TM patients tested positive for enteroviruses on swabs, but the significance is unknown.
Treatment data
Table 3 presents the treatment courses for all patients enrolled in the CAPTURE study. Treatment data were available for 51 AFM patients and 39 TM patients. From table 3, it can been seen that all patients received either intravenous immunoglobulin or corticosteroids as their initial treatment and no patients received PLEX as their initial treatment. Table 4 presents the parent/patient-recorded degree-of-improvement after initial treatment for TM and AFM patients. Based on the results of the ordinal regression model, there is no evidence that initial treatment with intravenous immunoglobulin resulted in greater improvement after initial treatment relative to initial treatment with corticosteroids (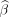 = −0.08, SE=0.57, p=0.89). Of note, we are unable to quantify the impact of corticosteroids or intravenous immunoglobulin on patient outcomes as a ‘no-treatment’ comparator arm was not available in this study.
= −0.08, SE=0.57, p=0.89). Of note, we are unable to quantify the impact of corticosteroids or intravenous immunoglobulin on patient outcomes as a ‘no-treatment’ comparator arm was not available in this study.
Treatment history
Degree of patient-reported qualitative improvement after initial treatment; cells without sample sizes denote results obtained from all patients in the given cohort
Patient-reported outcomess and parent-reported outcomes and clinician-derived outcomes
Due to the inability to adequately model and assess changes in patient-reported outcomes and parent-reported outcomes, as well as clinician-derived outcomes, descriptive statistics are presented in the supplementary material in online supplemental eTables 3–5.
Discussion
The AFM variant of myelitis attracted significant attention in the USA during an outbreak in 2014 with subsequent outbreaks in 2016 and 2018.7 These outbreaks have coincided with Enterovirus D68 circulation.8–11 Of note, the cohort described in this manuscript is demographically similar to previously reported AFM cohorts within the USA.12 Because the likely cause of AFM is the EVD68 virus, concerns relative to the use of corticosteroids in these patients have existed.13 Data from this prospectively studied cohort suggests that corticosteroids may be safe in these patients and may be of benefit to AFM patients with white matter.
To date, reported outcomes have been retrospective while the CAPTURE study was initiated prior to the recognition of AFM outbreaks and was, hence, able to prospectively collect data on this important patient population. This paper presents the demographic, clinical, radiographic, laboratory and outcome data of this cohort. Fifty-three percent of AFM patients tested with the respiratory enterovirus/rhinovirus PCR were positive, but subtyping was not universally available. Radiographic data revealed two patterns of interest. First, there was a predilection for the cervical spinal cord with the majority of patients having involvement of the C3–C5 region. Twenty-four per cent of patients had both upper and lower extremity weakness at onset. Biologically, if pathological changes in the cord were restricted to the cervical cord, lower extremity weakness would have to occur as a result of damage to white matter based corticospinal tracts. Thus, MRIs were reviewed and classified as grey matter restricted vs mixed grey and white matter changes. Approximately half of patients had evidence of mixed grey and white matter pathology. There are several potential explanations for these two patterns on MRI. While there could be more than one viral aetiology for AFM, there could also be an evolution of MRI findings over time that was not captured in isolated clinical MRIs.
Epidemiologic and animal model data both support the theory that AFM is the result of a viral infection causing death of anterior horn motor neurons.14 EVD68 is likely causative in the majority of cases.12 The recognition of two radiographic patterns might suggest that some patients suffer weakness from viral mediated motor neuron cell death while other patients have concomitant damage to surrounding white matter tracts. This damage could result from the inflammatory response elicited by the viral infection. Initial recommendations from the CDC counselled against the use of corticosteroids or PLEX in AFM out of concern for potentiating an infectious aetiology and worsening outcomes. Furthermore, animal model data documented fatality among mice treated with corticosteroids.14 Our data did not identify a unique risk to using corticosteroids in these patients and suggest that some patients may have experienced improvement. A controlled, randomised trial would be the ideal mechanism for quantifying risks and benefits, but due to the rarity and nature of this condition, such a trial would be difficult to execute.
Limitations
The CAPTURE study was not designed as a randomised control trial and was, therefore, at the mercy of patients’ treating physician. This fact introduced considerable heterogeneity in the treatment regimen of patients making comparison of patient-reported, parent-reported and clinician-reported outcomes difficult. Furthermore, due to the inclusion criteria of the CAPTURE study allowing for patients to enrol up to 6 months after onset, considerable data were missing corresponding to onset and 3 months postonset. This restricted analysis of longitudinal outcome measures due to the absence of baseline severity, which is crucial to the analysis of improvement over time. Furthermore, missing data due to patients’ lack of adherence and clinicians’ failure to capture all necessary data resulted in the inability to adequately examine patients’ progression after follow-up. Regarding missing data due to adherence, there is the risk that the missingness is due to the impact of the disease and missing not at random. That is, if missing scores were obtained, they would have been lower (or higher) than those that were observed. Lastly, in dealing with a paediatric population, almost half of which is less than 5 years old, many paediatric outcome measures (PROMIS Parent Proxy and PROMIS Paediatric form) are not validated, resulting in substantial missing data. Finally, the design of the study did not allow us to routinely collect accurate baseline clinical data, preventing certain analyses about relative change over time, particularly during the early post-treatment patient experience. A early worsening of symptoms after initial treatment with corticosteroids or intravenous immunoglobulin would not be accurately captured in this data. Furthermore, given the small number of patients with in person data, clinician derived data could not be used to analyse for disparate outcomes among treatment groups. Despite these limitations, the CAPTURE study was able to collect the first prospective clinical, radiographic, laboratory and outcome dataset for AFM, which has become a new public health concern in the USA.
Conclusions
The CAPTURE study prospectively enrolled patients and identified two distinct MRI patterns of spinal cord involvement. The dataset also failed to identify a significant difference between subjects receiving corticosteroids and/or PLEX versus intravenous immunoglobulin only, but our data raise the question of using corticosteroids and/or PLEX in certain patient cohorts. Prospective studies need to be continued and expanded to develop validated treatment algorithms for this growing public health concern.
Data availability statement
Data are available on reasonable request. Data can be provided to researchers with IRB approved/exempt protocols on request and review.
Ethics statements
Ethics approval
Institutional Review Board approval was obtained at each of the enrolling centres, but based on approval from the University of Texas Southwestern (study number 0 12 014–077).
Acknowledgments
The authors wish to acknowledge PCORI for funding of the CAPTURE study. PCORI gave feedback on the design of the study, but PCORI did not have a role in the conduct of the study; collection, management, analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The Transverse Myelitis Association (now renamed the Siegel Rare Neuroimmune Association) was instrumental in the recruitment of patients. Study data were collected and managed using REDCap electronic data capture tools hosted at The University of Texas Southwestern. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages and (4) procedures for data integration and interoperability with external sources. Finally, we wish to acknowledgement the patients and families who consented to share their data. BMG and MM had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors BG contributed to design, execution and analysis of the research data. PP, JD, AD, SH, CP, AR, TS and EAY contributed to the collection and analysis of the research data. MM, GC and CK contributed to the analysis of data and manuscript preparation.
Funding This work was supported by the Patient Centred Outcomes Research Institute (PCORI) grant number 1304–7079.
Competing interests BG is an unpaid board member of the Siegel Rare Neuroimmune Association. BG has received grant support from the Siegel Rare Neuroimmune Association and PCORI. CK is a paid employee of the Siegel Rare Neuroimmune Association.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.