Article Text
Abstract
Background New-onset refractory status epilepticus (NORSE) refers to patients without a previous history of seizures who have refractory status epilepticus for at least 72 hours without an identified aetiology. Despite the severe neurological sequelae of NORSE, little is known about this condition in paediatric patients.
Objective To describe the profile of paediatric patients with NORSE, the profile of seizures, possible causes attributed to this condition, treatments offered to patients and the outcomes at discharge from the paediatric intensive care unit (PICU).
Methods This retrospective, multicentre, descriptive study (case series) was conducted in the PICUs of three tertiary hospitals. We reviewed the medical records of all patients aged 0–16 years admitted to the participating PICUs between December 2013 and December 2017 with refractory status epilepticus, without a previous history of seizures or neurological disease.
Results Fifteen patients (2.4%) had NORSE. The median age of patients was 62.3 (IQR 26.2–75.4) months. All patients experienced prodromes before progressing to refractory status epilepticus. Twelve patients (80%) had fever up to 24 hours before seizures. NORSE was classified as cryptogenic in 66% of patients. Twelve patients were treated with complementary therapies, in addition to anticonvulsants. There was no standardisation in the treatment of patients. The overall mortality rate was 20%.
Conclusions NORSE is associated with high morbidity and mortality, without an identified aetiology in most cases and with a wide range of proposed therapies.
- autoimmune encephalitis
- epilepsy
- clinical neurology
Data availability statement
Data are available upon reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
What is already known on this topic
Summarise the state of scientific knowledge on this subject before you did your study and why this study needed to be done.
New-onset refractory status epilepticus (NORSE) is defined as a condition, not a specific diagnosis, with new onset of refractory status epilepticus without a clear cause in a patient without active epilepsy.
What this study adds
Summarise what we now know as a result of this study that we did not know before.
It can be devastating in a child and the cause after exhaustive investigation in 50% remain unexplained.
The therapies used were not standardised and included corticosteroids, immunoglobulins, immunosuppressants or ketogenic diet.
How this study might affect research, practice or policy
Summarise the implications of this study.
NORSE is a condition of high morbidity and mortality. Therefore, it is suggested that this diagnosis of NORSE be considered within 72 hours of refractory status epilepticus with no defined cause and that existing treatments be initiated at this time. An extensive diagnostic investigation can determine an autoimmune influence on its aetiology, leading to a specific treatment.
Introduction
Seizures are a medical emergency and a common reason for paediatric care.1 In most cases, the cause of seizures can be identified within 24 hours of diagnostic workup. However, in up to 20% of cases, this workup may not be conclusive, requiring immunological tests and other imaging tests that can delay treatment initiation.2
The severity of seizures and the risk of irreversible neurological sequelae increase with seizure duration. Refractory status epilepticus (RSE) is a severe form of seizure, lasting more than 30 min after treatment with two different classes of anticonvulsant drugs.3 4 Some patients with RSE may present with new-onset refractory status epilepticus (NORSE), which is characterised by the absence of a structural, metabolic or toxic cause that can explain the presence of RSE in otherwise healthy patients, despite extensive diagnostic workup and no control of status epilepticus within 48 hours.5 NORSE cases can, therefore, include patients with viral or autoimmune causes who progress to RSE without adequate seizure control. If no cause is identified after extensive investigation, it will be considered NORSE of unknown aetiology or cryptogenic NORSE.2
Several drug classes are used to control status epilepticus. However, there is currently no specific treatment for NORSE.5 In addition, treatments offered to patients with NORSE vary widely among services worldwide.6 These treatments are often expensive and their real benefits remain unclear in this patient profile. In paediatrics, studies on the treatment of children diagnosed with NORSE are still scarce, with no standard protocols for the management of these patients.4 7
The purpose of this study was to describe the profile of paediatric patients who develop NORSE, the characteristics of seizures and their outcomes during hospitalisation. We also investigated possible causes, treatments and outcomes of these patients at discharge from the paediatric intensive care unit (PICU).
Materials and methods
This retrospective, multicentre, descriptive study (case series) was conducted in the PICUs of three tertiary hospitals in southern Brazil, which are centres of excellence for the care of patients with neurological diseases.
We reviewed the medical records of all patients aged 0–16 years admitted to the participating PICUs between December 2013 and December 2017 who had RSE as the reason for admission to these units. The search was performed by means of electronic medical record review using the International Classification of Diseases—10th Revision (ICD-10) codes recorded at the time of patient admission to the PICU. The following ICD-10 codes were used in the search (all codes refer to pathologies that may contain RSE as a clinical presentation): G 049, G 40.5, G 040, G 048, G 400, G 401, G 402, G 403, G 404, G 405, G 408, G 409, G 410, G 411, G 412, G 418, G 419, A 86, A 858 and B 020.
After running this first search through all electronic medical records, we reviewed the retrieved records individually to include patients without a previous history of afebrile seizures, neurological diseases or any other disease that could explain the presence of RSE. Patients with a previous history of febrile seizures were included in the study, as this is a benign condition rather than a neurological disease. These inclusion criteria were based on the consensus definition of NORSE proposed by Hirsch et al.8 The only exclusion criterion was missing data in the medical record that would make it impossible to characterise the patient. The time to definitive diagnosis of NORSE ranges from 24 hours to 48 hours in the literature.5 9 We used the 72-hour interval to NORSE diagnosis because we consider it the optimal time to obtain the definitive results of some tests, such as toxicological screening, and the definitive reports of imaging tests. Patients with NORSE were also classified as having febrile infection-related epilepsy syndrome (FIRES) if they had a febrile infection starting between 14 days and 1 day before RSE onset.10
The demographic variables were sex (female or male) and age (in months). The clinical characteristics included the prodromes of RSE and patients diagnosed with FIRES associated with NORSE. We analysed the results of the following tests: cerebrospinal fluid (CSF), electroencephalogram (EEG), autoantibodies and MRI. Regarding comorbidities, we analysed length of PICU stay, days of mechanical ventilation (MV), number of intermittent anticonvulsants at discharge and NORSE-related complications and comorbidities. We also described the treatments performed and patients’ response to them.
Regarding EEG changes, continuous EEG monitoring was not available in any of the hospitals under study. To standardise this evaluation, we analysed the first EEG performed on PICU admission and the last EEG before PICU discharge. The antibodies investigated were anti-GAD, anti-NMDA, anti-AQP4 and antivoltage-gated calcium channel antibody. Mortality risk was assessed using the Pediatric Index of Mortality 2 (PIM2). PIM2 scores refer to the per cent risk of death of the children on PICU admission.11
Regarding the treatment used to control seizures, we analysed the duration of continuous infusion medications used in paediatrics for this purpose, such as midazolam, thiopental and ketamine.12 We also described second-line treatments for status epilepticus, such as immunosuppressive therapy and the ketogenic diet. We used the number of intermittent anticonvulsants as a criterion for neurological morbidity at PICU discharge.
Categorical variables are presented as numbers and percentages. Data are expressed as mean (SD) for continuous variables with normal distribution and as median (IQR) for continuous variables with skewed distribution.
Results
General characteristics
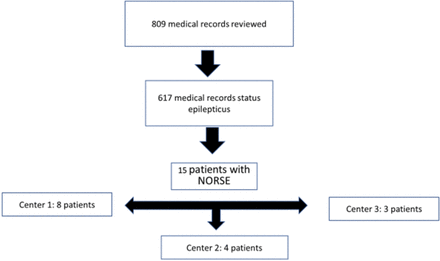
A total of 809 medical records were analysed according to the predetermined ICD-10 codes. Based on this analysis, 192 patients were not included because they did not have status epilepticus on PICU admission, and 617 were children who had RSE or other forms of seizure. Of these, 15 (2.4%) met the inclusion criteria and consisted of our sample of patients with NORSE (figure 1). No patient was excluded. Eleven patients were men and 14 were otherwise healthy patients. Only one child had chronic disease (type 1 diabetes mellitus), with the disease under control on the onset of NORSE. The median age of patients was 62.3 (IQR 26.2–75.4) months. Three of the 15 patients died, with an overall mortality rate of 20%.
Patient selection. NORSE, new-onset refractory status epilepticus.
Symptoms and initial assessment
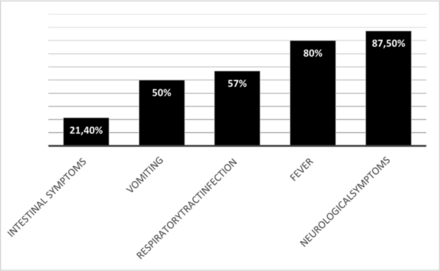
All patients experienced prodromes before status epilepticus. Figure 2 shows the patients’ symptoms before seizure onset. Some patients had more than one symptom during the diagnostic workup. CSF samples were collected from 13 patients, of whom 4 (30.7%) had altered cellularity characterised by pleocytosis with a predominance of polymorphonuclear cells. In only one of the patients with abnormal CSF results, cultures had a predominance of lymphomonocytes and were positive for herpes simplex virus.
Accumulated prodromes of patients with NORSE. NORSE, new-onset refractory status epilepticus.
Aetiology and autoantibodies
Regarding the aetiology of NORSE, antibody testing was performed in six patients (40%), and four of them (66.6%) had positive antibody responses. These four patients accounted for 26.6% of the sample and were classified as having autoimmune NORSE. Three of these patients (75%) had positive anti-GAD antibody; one of them, in addition to anti-GAD antibody, had positive anti-AQP4 antibody. The other patient had positive anti-NMDA antibody. Ten patients (66.6%) were classified as having cryptogenic NORSE. As previously described, only one patient had a confirmed viral aetiology (herpes simplex virus).
FIRES
Ten patients (66.6%) had FIRES, with a male-to-female ratio of 2.3:1. Three patients with FIRES belonged to the group of patients with autoimmune NORSE. Among the three patients who died, two had FIRES, with a mortality rate of 28.5% in patients with FIRES against an overall mortality rate of 20%.
EEG and MRI
Fourteen patients had an EEG performed. Only one patient did not have an EEG due to difficulty in performing it at the bedside. This patient died after 6 days; therefore, this child did not have an EEG recording during PICU stay.
Eleven patients (73.3%) underwent brain MRI, 9 of them (81.8%) had MRI abnormalities suggestive of a non-infectious inflammatory process and some degree of cerebral oedema. These changes in the initial MRI were not necessarily associated with a worse neurological outcome, since children with unaltered MRI had important neurological sequelae, such as poorly controlled seizures and levels of coma similar to those of patients who had abnormal MRI findings.
Clinical outcomes and PIM2
The median length of PICU stay was 42 (IQR 9–60) days. All children needed MV to manage status epilepticus, with a median of 17.5 (IQR 5–29.2) days of MV. Two children (13.3%) required tracheostomy due to prolonged MV, which was indicated after more than 30 days of invasive ventilatory support.
PIM2 was used in 13 patients, with a median PIM2 score of 0.05 (IQR 0.01–0.07). The expected mortality in our sample was 1.08, and the calculated standardised mortality ratio was 2.77. That is, the mortality was higher than expected as calculated by the PIM2 mortality score. As for comorbidities at PICU discharge, four patients (26%) were fed through a gastrostomy or nasogastric tube. Among the patients who were discharged from the PICU, 5 (33%) were unable to communicate verbally. Some neurological sequelae were recorded in 100% of cases, characterised by the use of anticonvulsants, persistent seizures and delay or regression of neuropsychomotor development, such as swallowing disorders, motor deficit and speech deficit.
Anticonvulsant and complementary therapies
The median duration of the use of continuous infusion anticonvulsant drugs was 12.5 (IQR 5.25–31.2) days. All patients used midazolam, ketamine and thiopental. The duration of use of each of these medications was not analysed separately. Patients used a median of 3 (IQR 2–6) intermittent anticonvulsants at PICU discharge. The most commonly prescribed drugs were phenytoin, phenobarbital, valproic acid and levetiracetam.
Regarding complementary therapies, eight patients (53.3%) received pulse therapy with methylprednisone for 3 days. The median time from the onset of status epilepticus to initiation of this treatment was 11 (IQR 6–17.5) days. One of these patients showed improvement, characterised by a reduction in the dose of anticonvulsants within 48 hours of the end of corticosteroid infusion.
Seven patients (46.6%) continued to have status epilepticus despite corticosteroid therapy and received immunoglobulin treatment. The median time from the onset of status epilepticus to immunoglobulin infusion was 18 (IQR 14.5–25.5) days. Two of these patients showed a decrease in the frequency of seizures according to EEG, as recorded in their medical records in the first 48 hours after the end of infusion. The other five patients did not have this specific information recorded in their medical records, but there was a reduction in the doses and number of continuous infusion anticonvulsants within 5 days of the end of this treatment.
Three patients (20%) were treated with the ketogenic diet. Two of them did not undergo any other complementary treatment. One of them received pulse therapy and immunoglobulin treatment in addition to the ketogenic diet as a second-line treatment. Two of these three patients had negative antibody tests; antibody testing was not performed in one patient. The median time from the onset of status epilepticus to treatment initiation was 25 (IQR 20–28.5) days. There were no records of seizure improvement with this treatment. One patient had a metabolic disorder, with hypercholesterolemia and need to discontinue treatment after 5 days.
Three patients (20%) received immunosuppressive treatment: rituximab was used in two patients and cyclophosphamide in one patient. Two of them had previously received immunoglobulin treatment, but with no significant seizure improvement. One patient was treated only with immunosuppressants. All three had positive antibody responses and showed improvement, with a reduction in the frequency and severity of seizures. The median time from the onset of status epilepticus to initiation of immunosuppressive treatment was 47 (IQR 39.5–50.5) days.
Four patients had no records of receiving complementary therapy. Of these, two died, one developed NORSE secondary to viral encephalitis (herpes simplex virus) and was treated with acyclovir and continuous and intermittent infusion of anticonvulsants, and one was treated with continuous infusion of intermittent anticonvulsants.
Discussion
Our study is one of the few to describe NORSE, a condition with high mortality that can lead to significant disabilities in survivors, such as poorly controlled epilepsy and cognitive-behavioural disabilities. The prevalence of NORSE in our sample was 2.5 times higher than that reported by Jafarpour et al5 and the most of cases that were tested (4 out of 6 patients) were considered of autoimmune aetiology, with an overall mortality rate of 20%. Among those who survived, none was discharged from the PICU without neurological sequelae or technology dependence.
We found a higher prevalence of NORSE in men (73%) as well as in children with FIRES.2 Studies in adults have shown a slight female predominance of NORSE (54.4%).9 In these studies, in general, NORSE and FIRES are classified as distinct clinical syndromes. In the present study, FIRES was included as a clinical presentation of NORSE,8 rather than a distinct syndrome, which may explain the male predominance in all cases of NORSE in our sample.
The mortality of patients with NORSE who have FIRES is 11–15%.13 14 In the present study, the mortality rate in patients with FIRES (28.5%) was similar to the overall mortality rate, which was 20%.
The age of onset of NORSE varies considerably, including a wide age range from infants to schoolchildren. Therefore, age and sex are not risk factors for the onset of NORSE.8
Regarding the signs and symptoms of NORSE, prodromal fever was more frequently observed in patients with positive than in those with negative antibodies (cryptogenic NORSE).6 Patients with autoimmune NORSE may show an exacerbated inflammatory response compared with patients with cryptogenic NORSE, which would explain the higher frequency of fever in these patients.2
Regarding the changes in CSF observed in the present study, they did not contribute to the diagnosis of NORSE.9 Nevertheless, collecting CSF samples remains essential to rule out causes that may explain status epilepticus, such as infectious diseases and neoplasms.9 One patient with CSF compatible with encephalitis who was positive for herpes simplex virus had clinical features that could not distinguish this patient from those diagnosed with NORSE.
EEG is a non-invasive graphic recording method used to diagnose seizures and to assess their characteristics.15 Studies on NORSE describe several types of seizures.16 Patients who had an EEG recording during PICU stay showed different changes, some of which were suggestive of encephalitis. Thus, we were unable to establish a pattern of EEG changes in NORSE. EEG is an ancillary tool to assess the frequency of seizures and response to established treatments. It is, therefore, important to use continuous EEG monitoring whenever possible, as it allows us to adjust the medications and to modify the treatment in real time. In our sample, as in many low-resource settings, continuous EEG monitoring was not available. In this case, for monitoring purposes, we suggest performing EEG recordings daily or whenever there is a change in the patient’s neurological status.
The underlying aetiology of NORSE can be identified in almost half of patients.5 The autoimmune aetiology is the most common underlying cause, with a prevalence ranging from 19 to 25%.5 14 16 The prevalence of autoimmune NORSE found in the present study (26.6%) might have been higher if antibody testing had been performed in all patients and if a larger panel of antibodies had been used.17 In addition, despite differences in terminology, there is evidence that cryptogenic NORSE and autoimmune NORSE are similar in most clinical and laboratory aspects, including the CSF profile.16 Therefore, it is possible that some cases of cryptogenic NORSE would correspond to autoimmune NORSE if a comprehensive analysis of the CSF antibody profile was performed.8
We found a high rate of MRI changes suggestive of an inflammatory process and some degree of diffuse cerebral oedema (81.8% of patients). Non-specific MRI signs, such as brain volume loss and signal hyperuptake (suggestive of an inflammatory process) in some MRI scans, especially when performed later in the course of the disease, are common in patients with RSE or poorly controlled seizures. This results from prolonged neurological insult, regardless of the aetiology of the seizures.5 18 In addition, some patients with few MRI abnormalities had a poor neurological prognosis. Therefore, MRI can be used to assess the extent of brain damage secondary to RSE in children with NORSE, but it does not necessarily serve as an indicator of neurological and functional prognosis in children with this disease.
Treatments performed with continuous infusion anticonvulsants were found to be insufficient to control seizures in cases of NORSE in our sample, which is consistent with data from the literature.7 Historically, they have played a key role as a neurological support measure in reducing neuronal insults until other treatments are initiated and achieve their therapeutic goals.19 Our data showed that there was no standard for the initiation and type of complementary treatment (corticosteroids, immunoglobulins, immunosuppressants or ketogenic diet) in patients with NORSE. In general, we observed that these therapies were introduced late. Studies evaluating early immunotherapy, for example, initiated within 72 hours of status epilepticus, have shown better neurological outcomes.16 20 Recently, the role of interleukins, especially the IL-1 receptor antagonist, has been studied. In this context, Anakinra medication has shown promising results.21 Because it is a rare condition, planning and conducting randomised clinical trials is difficult. Thus, studies are limited to case series and observational studies that do not standardise treatments, making it difficult to compare treatments.
There was no record of a decrease in seizures with the use of the ketogenic diet in our sample. This old treatment modality has been used as a therapeutic alternative in RSE because of its direct anticonvulsant effects resulting from the action of ketone bodies on the central nervous system. Ketone bodies promote increased levels of gamma-aminobutyric acid, with a consequent reduction in brain excitatory mechanisms. As a potential benefit in patients with NORSE, the ketogenic diet would act as an anti-inflammatory mechanism, associated with a reduction in the plasma levels of proinflammatory cytokines.19 22
We found no previous studies that have validated prognostic scores, such as PIM2, in NORSE. Our observed mortality was higher than the expected mortality as calculated by PIM2. Prognostic scores are designed to be used in large populations with mixed cases. However, these scores, such as PIM2, can sometimes be used from an individual point of view for prognostic purposes. This was not the case in the present study. In our sample, the PIM2 score underestimated the severity of NORSE. Further studies involving a larger number of patients are needed to validate these findings. As for mortality (20%), this rate is consistent with the literature, which shows rates around 25%.5 23
NORSE is a rare clinical presentation associated with high morbidity and mortality, which motivated us to conduct this case series. At the present time, we have many questions and few answers about this disease. New studies are needed to fill these knowledge gaps, so that in the future, we can modify its current unfavourable prognosis. Our study has some limitations that need to be considered. First, our data collection was based on an initial search for patients using ICD-10 codes, so it is possible that some cases of NORSE were not identified by this search strategy. Second, treatments, approaches and diagnostic tests varied widely, thus precluding a more in-depth comparative analysis. Finally, not all patients underwent a complete diagnostic workup, with antibody testing, and none underwent continuous EEG monitoring. Addressing these limitations could provide important information to this case series.
Conclusion
NORSE is a rare condition characterised by RSE and associated with high morbidity and mortality in the PICU setting. Its prevalence may be underestimated due to a lack of knowledge of the condition among intensivists. Given the absence of characteristic signs and symptoms or specific tests for this disease, clinical diagnosis and absence of a clear aetiology for the occurrence of RSE after the initial investigation are the main diagnostic criteria. Autoantibodies are often present, and treatment varied widely among the study centres. There is no therapy of choice, and the vast majority of patients who survive, if not all of them, are likely to be discharged from the PICU with important neurological and functional sequelae.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
The main institution is affiliated with the Pontifical Catholic University of Rio Grande do Sul, Brazil and authorised the study after approval by the Research Ethics Committee, under Ethics Approval Certificate number 04345118.5.10015336.
Acknowledgments
This research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
References
Footnotes
Contributors ALTdA is the contributor statement and guarantor. PCRG contributed to the conception or design of the study and acquisition, analysis or interpretation of the data; drafted the manuscript; critically revised the manuscript; gave final approval; and agree to be accountable for all aspects of work ensuring integrity and accuracy. CTT and CADC contributed to the acquisition, analysis or interpretation of data; critically revised the article; gave final approval; and agree to be accountable for all aspects of work ensuring integrity and accuracy. GRHA, FC, ARL, FB, GE, JP and PC contributed to the conception or design of the study, critically revised the manuscript, gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Funding This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Finance Code 001.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.