Article Text
Abstract
Background Intramuscular injections of botulinum toxin A (BTX-A) have been used in the treatment of sleep bruxism (SB) however controlled trials are limited and the optimal injection strategy and dose is not known.
Methods This double-blind, randomised, placebo-controlled, cross-over study evaluated the efficacy and safety of BTX-A in participants with SB. Average bruxism events per hour of sleep (Bruxism Index, BI) was calculated using surface electromyography. Participants with BI >5 were included and randomised by order of injection (active or placebo with the opposite 20 weeks later) and into one of three differing treatment groups: bilateral masseter (60 units(U)), bilateral masseter and temporalis (90U) and bilateral masseter, temporalis and medial pterygoid muscles (120U). Change in BI and subjective measures of headache, pain, and bruxism at 4 and 12 weeks was calculated following intervention, and differences between treatment groups analysed.
Results 41 participants were recruited, 35 randomised and data from 22 participants (14 female) were analysed. BI was significantly lower at 4 weeks after active treatment when compared with placebo (mean=−1.66, p=0.003), not sustained at 12 weeks. The difference was greater with higher doses injected and among those with greater baseline BI. There was no difference in subjective measures at any time point. Five participants injected had mild, transient side effects.
Discussion Targeted BTX-A injection is a safe and effective treatment for SB. A greater benefit may be achieved by administering BTX-A into more muscles and at higher total doses and among those with higher baseline BI.
Trial registration number ACTRN12618001430224.
- BOTULINUM TOXIN
- SLEEP DISORDERS
Data availability statement
Data are available on reasonable request.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
WHAT IS ALREADY KNOWN ON THIS TOPIC
Botulinum-toxin-A (BTX-A) is used in the treatment of sleep bruxism (SB), with varying doses and muscles targeted.
Controlled studies and objective evidence of efficacy is limited.
WHAT THIS STUDY ADDS
This controlled, double-blinded, cross-over study confirms the safety and efficacy of BTX-A in the treatment of bruxism.
Expanding the injections to include masseter, temporalis and medial pterygoid muscles is a safe and effective approach.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Larger studies are required to evaluate the optimal injection paradigm for the treatment of SB.
More than one treatment cycle is likely to be required to fully assess efficacy of BTX-A and to identify the optimal injection sites for treatment response. Further studies are required to establish optimal outcome measures in SB, including those that best reflect subjective outcomes.
Introduction
Bruxism is a repetitive masticatory muscle activity characterised by clenching or grinding of the teeth and/or bracing or repetitive thrusting of the mandible, occurring while awake, asleep (sleep bruxism, SB) or both.1–3 Bruxism can cause morbidity in the form of abnormal tooth wear, grinding noises and jaw or more widespread craniocervical discomfort and pain.1 4 Bruxism common, however, prevalence has been difficult to establish, at least in part due to the variable nature of the condition within affected individuals and the inherent difficulty in defining ‘abnormal’ function from normal physiological function.5 6 In the largest epidemiological study to date, prevalence in the general population was 5.5%–7.4% depending on criterion used.6 The aetiology and pathophysiology of bruxism is not fully understood but it is likely to be multifactorial, with contributing factors varying among affected individuals. Both peripheral and central neurological contributions have been reported in otherwise well individuals, including alterations in dopaminergic pathways, sleep arousal and psychological factors.4 7–9 Bruxism can also occur in association with particular neurological disorders (eg, craniocervical dystonia, Huntington’s disease, cerebellar disease,1 2 graft-versus-host disease10) and secondary to centrally acting medications (eg, selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors, barbiturates, phenethylamines and some opiates.11
Despite the prevalence and morbidity associated with bruxism, effective treatment options are lacking. Current treatment modalities include dental occlusal splints,12–14psychobehavioural therapies12 14 and systemic pharmacological therapies (eg, benzodiazepines and dopaminergic therapies),12 all of which have demonstrated limited efficacy. More recently, targeted botulinum toxin A (BTX-A) has emerged as a potential therapy, with most but not all studies demonstrating benefit, including three small placebo-controlled trials.2 15–18 Despite its subsequent off-label use for this indication in several regions, current evidence does not adequately guide the use of BTX-A for the treatment of SB, in particular, the most effective and safe dose and pattern of BTX-A injection has not been evaluated. Evidence, to date, has been particularly limited by small sample sizes, significant variability in doses, injection paradigms and outcome measures between studies.
As such, the primary aim of this double-blind, randomised, placebo-controlled, cross-over study was to evaluate the efficacy and safety of targeted injections of BTX-A in participants with bruxism. We further investigated the optimal treatment strategy and doses, duration of benefit, and factors associated with benefit following BTX-A injection.
Methods
Participants
Participants were recruited between 2017 and 2020 from Royal Melbourne Hospital Neurology Clinics, Sleep and Respiratory Medicine Clinics, Dental practices and through participant self-referral following a diagnosis of bruxism by an external dentist or physician. Participants aged 18–80 years were included if they fulfilled a diagnosis of bruxism according to the International Classification of Sleep Disorders Revised (ICSD-R) criteria.19 20 Exclusion criteria were current use of medications affecting muscle relaxation (eg, benzodiazepines), a prior history of severe jaw trauma, concurrent orofacial pain of an alternate aetiology, a history of neuromuscular disease, contraindications to BTX-A (including current or planned pregnancy), and previous or current BTX-A injection for bruxism or other indications within 16 weeks of starting the study. Participants were also excluded if the baseline Bruxism Index (BI, described in detail below) was less than five.
Clinical evaluations
A detailed clinical history was obtained at baseline by a neurologist and participants were examined for physical features of bruxism: indentations in the inside of the cheeks or the edges of the tongue, hypertrophy of the masseter muscles, excessive dental wear (including loosening or fracturing of the teeth), temporomandibular joint clicking or locking. Participants were also assessed for a coexisting diagnosis of any primary headache disorder at baseline classified according to the International Classification of Headache Disorders second edition.21
Intervention
Participants each received two sets of targeted intramuscular injections (active treatment and placebo) separated by 20 weeks to allow full recovery between injections, with order of injections randomised (online supplemental figure 1). The active treatment was on a BTX-A (Botox, Allergan Australia) using a dilution of 100 mouse units (U) in 2 mL normal saline (NS); placebo injections were of an equivalent volume of 0.9% sterile NS. Participants were further randomised in a balanced configuration into three separate intervention groups, differing by injection paradigm and dose. The doses injected into temporalis and masseter were chosen based on previous publication reports of safety and efficacy,2 15–18 and our own experience. The dose of BTX-A injected into the medial pterygoid muscles was chosen based on our centre’s experience of favourable safety and efficacy profile. Group A were administered 30U BTX-A (or 0.6 mL NS) in a single site in each masseter muscle (total dose 60U). The anterior border and most prominent bulge of the masseter muscle was identified during jaw clenching, with injections inferior to a line connecting the inferior border of the ear lobe and angle of the mouth to minimise the risk of diffusion into the zygomaticus complex. Group B were administered 30U BTX-A in masseter (or 0.6 mL NS) and 15U BTX-A (5U in three sites (or 0.3 mL NS) in the temporalis muscles (total dose 90U) identified by palpation during jaw clenching. Group C were administered 30U BTX-A (or 0.6 mL NS) in masseter, 15U BTX-A (or 0.3 mL NS) in temporalis and 15U BTX-A (or 0.3 mL NS) into a single site in the medial pterygoid muscles bilaterally (total dose 120U). The medial pterygoid muscle was injected extraorally via a submandibular route with the patient lying supine with the neck extended. The needle was inserted medial to the mandibular angle and progressed parallel to the inside of the mandible to a depth of 10–15 mm. Active treatment and placebo injections were prepared by pharmacists who were blinded to the participants’ clinical data. Masseter and medial pterygoid injections were performed under 26 guage needle electromyography (EMG) guidance and temporalis injections performed using a 30 guage needle. All injections were performed by an experienced neurologist (LK or AE) who was blinded to the content of the injection (BTX-A or placebo) and to objective or subjective participant data.
Supplemental material
Outcome measures
The primary outcome was the difference compared with placebo in the severity of participants’ bruxism as measured by the BI (described below) at two time points, weeks four and 12 following injection.
Secondary outcomes included the differences among the intervention groups in the BI at weeks 4 and 12, and changes in subjective outcome measures between active and placebo treatments, as described below.
Surface EMG recordings
The BI represents the number of bruxism events/hour of sleep.22 Surface EMG (sEMG) of the masseter muscles bilaterally was recorded overnight using a two-channel portable sleep system (Nox-T3, Nox Health Group). To enable accurate home recording, subjects were first instructed and trained in the use of the equipment by an investigator blinded to the treatment arm. Nocturnal sEMG data were acquired for at least 6 hours per night for three consecutive nights. Participants were asked to commence their recording on retiring to sleep and were instructed not to wear an occlusive splint during sEMG recording nor record on a night following consumption of alcohol or other recreational drugs to avoid potential sEMG confounding. Raw data for all participants at baseline and all study timepoints was analysed by a single scientist (EW) who was blinded to the participant’s presenting symptoms, subjective rating scales (see below) and treatment paradigm. The number of times there was a tonic, phasic and mixed (tonic and phasic) masseter contraction was analysed and the average number of events/hour was calculated per night of recording (BI).22 The average BI (over all nights of recording) was then calculated for each time point and analysed.
Subjective rating scales
At baseline and weeks four and 12 following injection, participants were asked to complete a bruxism symptom questionnaire (five-point scale),19 23 the Short Form McGill (SFM) pain scale24 (indicating pain in the jaw, head and neck region), the Headache Impact Test-6 (HIT-6) questionnaire,25 and the Epworth Sleepiness Scale (ESS).26 The SFM included the subscales: Pain Rating Index (PRI), a present pain intensity Visual Analogue Scale (VAS) and a score indicating the overall intensity of pain experience (SFMIII). The total PRI was further subdivided into the affective PRI and sensory PRI.24
Adverse events
Adverse events were assessed and recorded at each study visit. In particular, side effects of bruising, weakness of chewing or swallowing, facial weakness and xerostomia were assessed.
Statistical analysis
Two-way repeated measures analysis of variance (ANOVA) was performed to compare the effect of BTX-A injection versus placebo in participants with bruxism over weeks 4 and 12 postbaseline. In a post hoc analysis, the effect of treatment was also compared between the treatment groups A, B and C. Only complete cases with all study time-points per patient and intervention were analysed. The data were analysed using jamovi V.1.6.14 program. Secondary analysis was performed using an analysis of covariance (ANCOVA) to evaluate the association between the baseline BI and the effect of BTX-A injection versus placebo.
Results
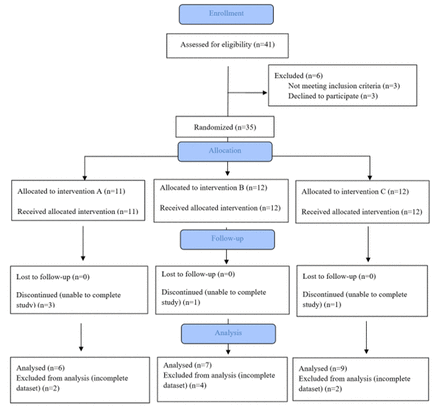
Thirty-five participants were recruited to take part in the study, however, 13 were excluded after commencement due to inability to complete the study or dataset collection (figure 1). Of the remaining 22 participants, 14 were female and 8 male. Participants ranged from 22 to 68 years old (mean 42.1 years). Six participants were randomised to group A, seven to group B and nine to group C (table 1). Coexistent headache disorders were common at the time of enrolment, with 18/22 participants having coexistent headache; 14 participants having episodic tension-type headache, four episodic migraine without aura and five episodic migraine with aura (7 participants had more than one headache type). The majority (18/22) of the participants included for analysis had trialled occlusive splints for symptom control.
Baseline participant data
CONSORT diagram.
Mean baseline BI for all participants was 8.29 (SD=2.88) (table 1) and there was no evidence of differences in baseline BI between the three treatment groups (F (2, 21)=1.59, p=0.25).
Active versus placebo intervention
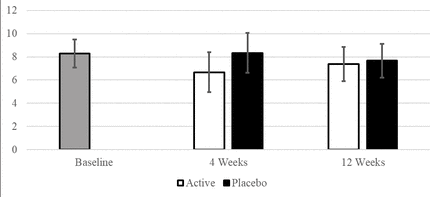
A two-way repeated measures ANOVA demonstrated a significantly different BI during the treatment phase, compared with the placebo phase (F (1, 21)=8.09, p=0.01). Further post hoc analysis showed that during the treatment phase, BI was significantly lower at 4 weeks postinjection when compared with placebo (mean difference=−1.66, p=0.003), but this difference was not sustained at 12 weeks (p=0.58) (table 2, figure 2).
Bruxism Index across time points
Change in Bruxism Index from baseline following botulinum toxin injection.
Analysis across intervention groups
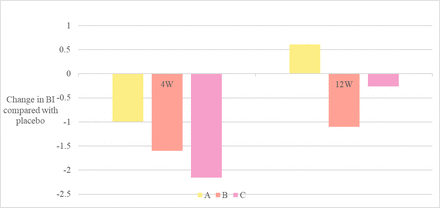
Secondary analysis demonstrated that Group C experienced the largest reduction in BI at 4 weeks post injection of BTX compared with placebo (see figure 3). Participants in this group experienced a mean reduction of 2.15 in BI (p=0.009). Groups A and B did not demonstrate a significant difference by the same measure (group A: p=0.287; group B: p=0.073), nor did we find evidence of difference among the treatment groups (F(2, 21)=2.11, p=0.15), and there were no differences in BI between active and placebo at the 12-week mark for any group.
Mean bruxism index by injection group.
Comparison of intervention groups
Secondary analysis (ANCOVA) demonstrated that group C experienced the largest reduction in BI at 4 weeks postinjection of BTX compared with placebo (figure 3). Participants in this group experienced a mean reduction of 2.15 in BI (p=0.009). The decrease observed in groups A and B was relatively smaller and did not reach the level of statistical evidence (group A: p=0 .287; group B: p=0.073). Further, we did not find evidence of difference in the BI change among the treatment groups (F(2, 21)=2.11, p=0.15), and there were no differences in BI between the active treatment groups and placebo at the 12-week mark for any group.
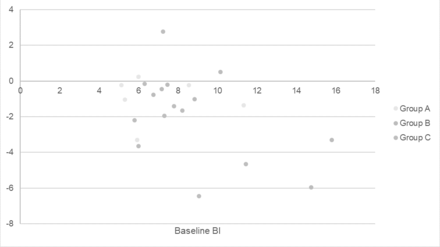
Further analysis demonstrated that participants who had a higher BI at baseline experienced a greater effect of BTX-A compared with placebo after 4 weeks compared with participants with a lower baseline BI (F(1, 21)=7.76, p=0.012); (figure 4).
Baseline Bruxism Index and change in Bruxism Index at 4 weeks compared with placebo.
There were no significant differences in the results of any subjective scales between the active and placebo phases at weeks four and 12 following injection, regardless of the dose of BTX-A injected (table 3).
Mean subjective rating across time points
Adverse events
Injections were well tolerated. Four participants developed transient painless weakness or chewing fatigue (two from group C, one from each of groups A and B); this was did not impact on function, lasted less than 2 weeks in all cases, and all those affected requested to continue open-label injections after study completion due to perceived benefit. One participant from group B developed mild weakness of the lower face following active treatment, lasting 10 days. Mild bruising occurred following one participant injection (group A), and another participant from group C terminated study involvement after the first dose due to injection discomfort.
Discussion
This study provides class II evidence for the efficacy and safety of targeted BTX-A injection in the treatment of SB, as measured objectively by sEMG. Comparison of different BTX-A doses and intramuscular injection paradigms demonstrates that bilateral masseter only (30U), masseter (30U)/temporalis (15U) and masseter (30U)/temporalis (15U)/medial pterygoid(15U) treatment regimens are comparable in terms of safety, and while our study was not powered to fully uncover between-group differences, there was a greater magnitude of effect among participants randomised to the injection paradigm with higher total dose and number muscles injected (ie, group C). The treatment was safe, with bruising over the masseter in one participant and only 5 out of the 35 participants experiencing mild short-lived focal weakness following injection with BTX-A.
While there was a reduction in BI following BTX-A injection at week 4, this did not persist at week 12, regardless of the dose of BTX-A or muscles injected. This wearing off of BTX-A effect is consistent with the biological effects of BTX-A, with gradual recovery of motor function usually 3–4 months after injection.27
In our cohort, the duration of effect of BTX-A was not significantly different despite an overall doubling of the dose and expansion of muscles injected between group A (60U total) and C (120U total). Between-group differences may have been uncovered with a larger sample size and longer follow-up period after repeated injections. The magnitude and duration of muscle weakness induced following BTX-A increases with repeated injection,28 and thus the degree and duration of effect of BTX-A on SB may increase with subsequent injections even with stable dosing. It is possible, however, that the beneficial effect of BTX-A on SB is not dose-dependent. By inducing a degree of masticatory muscle weakness, subsequent behavioural changes on affected individuals, exhibited by reduced clenching and grinding, may be independent of the degree of muscle weakness. Indeed, in measuring BI we are not measuring the strength of masticatory muscles, but rather the frequency and pattern of masticatory muscle activation.
We explored baseline participant characteristics associated with an objective response to BTX-A injections. The greater the baseline BI, the greater the objective change in BI following BTX-A injection at week 4, regardless of the dose injected (figure 3). This is an important and novel finding, and supports the use of objective measurements of bruxism severity in predicting benefit from BTX-A injection. Further still, participants with a higher baseline BI did not otherwise differ from the remainder of the group clinically or in baseline subjective measurements. In the absence of overnight EMG and BI determination, these more severely affected individuals would remain undifferentiated in clinical practice.
In considering how these findings compare with the effects of alternative treatments for SB, the most widely used and studied treatment for SB is oral splint devices. Objective benefit of splints on SB as measured by sEMG is limited to the immediate short-term (ie, the first nights of continuous use) but do not persist at weeks 2 onwards.13 29 It is widely understood that these devices will reduce dental destruction due to bruxism but not change the overall bruxing activity for sustained periods, and therefore, not reduce pain or headache associated with bruxism. Our study population reflects this; the majority (82.8%) of participants included in our analysis had previously or currently used dental splints.
We demonstrated a discrepancy between bruxism severity (measured by BI) and bruxism frequency (measured by questionnaires), with no subjective improvement reported at 4 and 12 weeks after injection. This is consistent with a prior study that demonstrated no significant change in the quantifiable portion of the Montreal Bruxism Questionnaire following BTX-A injection when compared with placebo, despite benefit measured by polysomnographic measures of bruxism and other subjective scales (a Clinical Global Impression and VAS of pain and bruxism overall).16 An explanation can be found in the nature of the bruxism symptom questionnaire, which asks the participant to indicate how often in the last month they think they bruxed at night, how often their sleeping partner thinks they bruxed at night and how often they woke with jaw stiffness, graded from zero (never) to five (every day). Specifically, although this clearly captures frequency of symptoms, the severity or morbidity of these symptoms is not reflected. Clinical evaluation and any future research evaluating BTX-A in the treatment of SB should therefore consider this observation when selecting appropriate outcome measures.
Similarly, we did not observe evidence for change in pain outcomes as measured by the SFM pain questionnaire (including sensory and affective subscores) or the VAS for pain. This was an unexpected finding and at odds with participants’ overall experience at the end of the study, with 24 (77%) requesting ongoing injection due to a perceived improvement in pain. Overall, changes to pain levels following BTX-A in SB have been conflictingly reported using SFM and VAS in other studies. For example, two studies using the VAS demonstrated change in jaw pain at week 4,16 30 while a separate study failed to find changes at this time point, instead finding a change at 6 months postinjection.17 Day-time sleepiness and headache-related disability following injection, as measured by the ESS and HIT score respectively, were not influenced by BTX-A injection at any dose evaluated in our study, replicating the observations of a prior placebo-controlled study.16 Taken overall, our findings suggest that current subjective outcome measures are less sensitive than objective measures for the purpose of evaluating treatment effect in SB. It is also possible that a longer period of bruxism treatment with BTX-A (for example with repeated injection) is required for bruxism-associated myofascial pain to improve with treatment; for example when Botox injections are used for prevention of chronic migraine, peak effect occurs after at least a second set of injections31 32 The best subjective outcome measures of SB and those most sensitive to change following intervention have not been established and need ongoing consideration in publications in the field.
The majority of participants had coexistent headache disorder, with 82% suffering either tension-type headache or migraine, and 32% having multiple types of headache. This exceeds the background estimated prevalence of these disorders.33 There are several potential contributors for this: there is an increased incidence of tension-type headache and migraine among those with SB.34 Referral bias is also possible, with those with concurrent headaches having greater disability and be more likely referred to a neurology centre for treatment. Importantly, however, the relatively high prevalence of headache disorders in our cohort may have reduced the sensitivity of our subjective outcome measures in detecting changes to bruxism frequency or severity, and thus contribute to the disparity between our objective and subjective measures of bruxism or bruxism related pain after active treatment.
Our study has several limitations. The small sample size may limit generalisability of these results, particularly in comparing individual treatment groups. On the other hand, this trial was sufficiently powered to unequivocally demonstrate efficacy of BTX-A for the treatment of bruxism. Concurrent EEG channel recordings would have confirmed that the recorded sEMG activity was truly occurring during sleep, however, the practicalities of such a system were beyond the scope of participants’ use at home. We feel that this has not significantly impacted study findings, as wakeful EMG activity can be distinguished from involuntary activity in sleep, and would be expected to occur equally at all time points in all participants. Due to randomisation and a cross-over design, we expect this potential noise to affect both intervention and placebo, and therefore, does not represent a bias. Finally, the techniques used in this study are accessible for more widespread use (as opposed to EEG monitoring), making these findings more applicable for clinical practice.
In conclusion, targeted BTX-A injection is safe and effective in the treatment for bruxism, as measured objectively by the BI. Those with more severe bruxism may derive a greater benefit from BTX-A injection and the use of overnight EMG recordings may assist in predicting a response following BTX-A. While a greater benefit may be achieved by administering BTX-A into a greater number of muscles (and therefore, at higher total doses), larger studies which include objective evaluation following repeated BTX-A injections are required to establish the optimal dosing of BTX-A in the treatment of SB.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Royal Melbourne Hospital Research Ethics Committee HREC2015.195. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
The authors wish to acknowledge the participants of this research.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @AndrewHEv
Contributors TD, LK and AE designed the study. BC, LK, AE and EW implemented the study. Data was collected by BC and CH. Statistical analysis was performed by CH, SS and TK. First draft manuscript was written by BC, with manuscript revisions performed by all authors. BC and LK are guarantors.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests There are no financial disclosures/conflicts of interest for any authors concerning this research. Financial disclosures unrelated to this research are as below: TK served on scientific advisory boards for BMS, Roche, Janssen, Sanofi Genzyme, Novartis, Merck and Biogen, steering committee for Brain Atrophy Initiative by Sanofi Genzyme, received conference travel support and/or speaker honoraria from WebMD Global, Eisai, Novartis, Biogen, Sanofi-Genzyme, Teva, BioCSL and Merck and received research or educational event support from Biogen, Novartis, Genzyme, Roche, Celgene and Merck. LK served on medical advisory boards and received speaker honoraria from CSL Behring. Andrew Evans reports reimbursement of travel expenses to scientific meetings or honoraria for lecturing or consultation from UCB, Teva, Stada, Allergan, Merz, Abbott and Abbvie, advisory board honoraria from Abbvie, Allergan, Stada and UCB, and holds shares in CSL and Global Kinetics Corporation. BC, TD, SS, EW and CH have no disclosures.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.